Abstract
Literature on non-ampullary–duodenal carcinomas is limited. We analyzed 47 resected non-ampullary–duodenal carcinomas. Histologically, 78% were tubular-type adenocarcinomas mostly gastro-pancreatobiliary type and only 19% pure intestinal. Immunohistochemistry (n=38) revealed commonness of ‘gastro-pancreatobiliary markers’ (CK7 55, MUC1 50, MUC5AC 50, and MUC6 34%), whereas ‘intestinal markers’ were relatively less common (MUC2 36, CK20 42, and CDX2 44%). Squamous and mucinous differentiation were rare (in five each); previously, unrecognized adenocarcinoma patterns were noted (three microcystic/vacuolated, two cribriform, one of comedo-like, oncocytic papillary, and goblet-cell-carcinoid-like). An adenoma component common in ampullary–duodenal cancers was noted in only about a third. Most had plaque-like or ulcerating growth. Mismatch repair protein alterations were detected in 13% (all with plaque-like growth and pushing-border infiltration). When compared with ampullary (n=355) and pancreatic ductal (n=227) carcinomas, non-ampullary–duodenal carcinomas had intermediary pathologic features with mean invasive size of 2.9 cm (vs 1.9, and 3.3) and 59% nodal metastasis (vs 45, and 77%). Its survival (3-, 5-year rates of 57 and 57%) was similar to that of ampullary–duodenal carcinomas (59 and 52%; P=0.78), but was significantly better than the ampullary ductal (41 and 29%, P<0.001) and pancreatic (28 and 18%, P<0.001) carcinomas. In conclusion, non-ampullary–duodenal carcinomas are more histologically heterogeneous than previously appreciated. Their morphologic versatility (commonly showing gastro-pancreatobiliary lineage and hitherto unrecognized patterns), frequent plaque-like growth minus an adenoma component, and frequent expression of gastro-pancreatobiliary markers suggest that many non-ampullary–duodenal carcinomas may arise from Brunner glands or gastric metaplasia or heterotopic pancreatobiliary epithelium. The clinical behavior of non-ampullary–duodenal carcinoma is closer to that of ampullary–duodenal subset of ampullary carcinomas, but is significantly better than that of ampullary ductal and pancreatic cancers. The frequency of mismatch repair protein alterations suggest that routine testing should be considered, especially in the non-ampullary-duodenal carcinomas with plaque-like growth and pushing-border infiltration.
Similar content being viewed by others
Main
Tumors arising in the non-ampullary (extra-ampullary) segment of the duodenum are considered true ‘duodenal cancers’. These primary ‘duodenal’ carcinomas are rare and constitute 35–50% of all small intestinal carcinomas.1 Their analysis is frequently combined with other small bowel, pancreatic, and distal bile duct cancers (periampullary cancers) that has caused marked confusion in the literature regarding their frequency and clinicopathologic characteristics.2 Through a more vigorous site-specific classification, many tumors that were previously deemed ‘duodenal cancers’ have now been recently shown to comprise distinct specific types, such as those arising from the ampulla of Vater (ampullary carcinoma of not-otherwise-specified type), those that grow predominantly on the ampulla’s duodenal surface (ampullary–duodenal or ‘periampullary’–duodenal),3 and those that arise from non/extra-ampullary–duodenum. It is also now being appreciated that ampullary carcinoma arising from the ampullary ducts, that is, ampullary ductal group, has distinct characteristics. Although ampullary–duodenal cancers are typically large, ulcerated, or vegetating intestinal-type adenocarcinomas that are often associated with an abundant intestinal-adenomatous component and have a relatively good survival (3-year survival 59%, and 5-year 52%), ampullary ductal cancers typically form scirrhous circumferential tumors on the wall of ampullary ducts (ampullary portion of common bile duct and main pancreatic duct) with minimal mucosal changes in the duodenal surface of the ampulla, and are typically pancreatobiliary type adenocarcinomas and show aggressive behavior despite being small (mean size 1.8 cm, and 5 year 29%).3 For non-ampullary–duodenal carcinomas, the literature reveals conflicting results, largely because they have often been analyzed with ampullary or other intestinal cancers. Recent studies are indicating that they may in fact be fairly similar to ‘ampullary cancers’,4, 5 but many of their characteristics have not been fully elucidated.
To better characterize the clinicopathologic features, immunophenotype and clinical behavior of non-ampullary–duodenal carcinomas (duodenal cancers that clearly spare the ampulla), we performed an analysis of 47 resected examples, and compared these with those of 355 ampullary and 227 pancreatic ductal adenocarcinomas.
Materials and methods
The study was conducted in accordance with the Institutional Review Board requirements. The study included the resected duodenal carcinomas collected from 2000 to 2014 from Emory University Hospital and University of Pittsburg. Non-invasive tumors (adenoma-only cases) and tumors arising in the setting of familial adenomatous polyposis and Crohn’s disease were carefully excluded. Using these criteria, 47 cases were qualified as non-ampullary–duodenal carcinomas. For comparison, 355 ampullary (of which, 29 were ampullary–duodenal, 66 ampullary ductal and the remaining 260 were either intra-ampullary papillary-tubular neoplasm—associated or not-otherwise-specified types) cancers and 227 pancreatic ductal adenocarcinomas were retrieved from the database during the same period of time, and assessed for long-term follow-up.
Definitions
A tumor was designated as:
-
1
Non-ampullary–duodenal carcinoma, if it histologically spared the ampulla, with no evidence of preinvasive or invasive lesions in the major ampulla.
-
2
Ampullary carcinoma, if its epicenter was located in the lumen or walls of the distal (intra-ampullary component) common bile duct and/or pancreatic duct, or at the ‘papilla of Vater’ (junction of duodenal and ampullary mucosa as defined by the College of American Pathologists),6 or the duodenal surface of the papilla.3
-
a
Ampullary–duodenal carcinoma, (also known as ‘periampullary–duodenal) were regarded as a subset of ampullary carcinomas arising from the duodenal surface of the ampulla itself, and showing an exophytic ulcero-fungating tumor growing into the duodenal lumen and eccentrically engulfing the ampulla orifice with only minimal intra-ampullary luminal involvement.3
-
b
Ampullary ductal carcinoma, represented carcinomas arising from nontumoral (flat) intraepithelial neoplasms of the ducts, and forming constrictive, sclerotic, plaque-like thickening of the walls of the common bile duct and/pancreatic duct resulting in mucosa-covered, button-like elevations of the papilla into the duodenal lumen.3 These are now regarded under the heading of ‘intra-ampullary’ category by the recent modification of the College of American Pathologists protocol.
-
a
Demographic and Clinical Data
Information on the patients’ gender, age, and clinical outcome was obtained from the medical records, by contacting the primary treating physician, or through the Surveillance and Epidemiology and End Result database.
Pathologic Parameters
These 47 cases were analyzed for ‘overall tumor size’, the size of the invasive carcinoma, the growth pattern, the presence of perineural and lymphovascular invasion, and the lymph node status, according to the American Joint Committee on Cancer staging tumor classification,7 the presence of preinvasive (adenomatous) components of the tumor and the features of the uninvolved mucosa at the tumor edges.
Histologically, cases were classified as tubular type (if the predominant pattern was tubular/glandular differentiation) and non-tubular types. The tubular group was subclassified into three categories: intestinal, gastro-pancreatobiliary, and mixed type (if the intestinal, pancreatobiliary, and/or gastric features were present in the same case).8 Briefly, invasive carcinomas with more basophilic appearance and complex glands lined by pseudostratified columnar cells with cigar-shaped nuclei were classified as intestinal type; those with widely separated small tubular units lined by one or two layers of cuboidal cells were classified as pancreatobiliary type; and those with a tubular and papillary proliferation with foveolar-type or pyloric-type (Brunner gland) differentiation were classified as gastric type.8 In this study, gastric and pancreatobiliary lineages were grouped together in accordance with recent concepts in pancreatic histology that favor the combined classification of these two lineages because of their close association, shared immunophenotype, and frequent co-occurrence.8, 9, 10 Non tubule-forming carcinoma types (such as mucinous, medullary, poorly cohesive/poorly differentiated carcinoma, and adenosquamous carcinomas) were classified according to the World Health Organization 2010 Classification of Digestive Tumors.11 Histologic findings of the carcinomas were noted for other patterns as well.
Immunohistochemical Analysis
Cell lineage markers
Immunohistochemical analysis was performed on 38 cases (with the tissue block or unstained slides available) with cell lineage markers, which are known to be differentially expressed in different components of the gastrointestinal tract: MUC1 (Clone Ma695, 1:160, Novocastra, New Castle, UK), marker typically present in pancreatobiliary differentiation; MUC2 (Ccp58, 1:100, Novocastra, New Castle, UK), intestinal (goblet cell) differentiation; CDX2 (CDX2-88, 1:200, Biogenex, San Ramon, CA, USA), intestinal transcription factor; MUC5AC (CLH2, 1:200, Leica), foveolar mucin marker; MUC6 (CLH5, 1:80, Leica), pyloric/Brunner gland marker; CK7 (OB-TL 12/30, 1: 40, DAKO, Carpenteria, CA, USA), typical of gastro-pancreatobiliary tumors; and CK20 (Ks 20.8, 1/40, Dako, Carpenteria, CA, USA) typically expressed in lower-intestinal neoplasms.
DNA mismatch repair protein markers
Immunohistochemical analysis was performed with MLH1 (G168-758, 1:20, BD Pharmiger, San Diego, CA, USA), PMS2 (A16-4, 1:50, BD Pharmiger, San Diego, CA, USA), MSH2 (FE11, 1:20, Calbiochem, San Diego, CA, USA), and MSH6 (44/MSH6, 1:50, BD Pharmiger, San Diego, CA, USA).
Methodology
Immunohistochemistry was performed using a polymer-based detection system (Envision+; Dako, Carpinteria, CA, USA) with mouse monoclonal antibodies according to the manufacturer’s instructions. Sections were deparaffinized and rehydrated with deionized water. Then, they were heated in citrate buffer, pH 6.0, using an electric pressure cooker for 3 min at 12–15 pounds per square inch at ~120 °C and cooled for 10 min before immunostaining. All slides were loaded onto an automated system (Autostainer; Dako), in which they are exposed to 3% hydrogen peroxide for 5 min, incubated with primary antibody for 30 min, incubated with labeled polymer (Envision+ dual link) for 30 min, incubated in 3,30-diaminobenzidine as a chromogen for 5 min, and counterstained with hematoxylin for 5 min. These incubations were performed at room temperature. Between incubations, sections were performed using the Tissue-Tek SCA cover slipper (Sakura Finetek USA, Torrance, CA, USA). Positive controls and negative controls with primary antibody replaced by Tris-buffered saline were run with the patient/study slides.
Evaluation of immunohistochemical stains
The percentage of cells showing cytoplasmic (MUC2, MUC5AC, MUC6, CK7, and CK20), apical membranous or cytoplasmic (MUC1), and nuclear (CDX2) labeling was evaluated. Only the cases with > 25% immunoreactive cells were regarded as positive.12 For mismatch repair protein status (MLH1, PMS2, MSH2 and MSH6), the lack of nuclear staining in the invasive carcinoma was interpreted as an abnormal result.
Statistical Analysis
Comparisons of patient and tumor characteristics were made by means of an unpaired Student's t-test for continuous variables and by χ2-analysis for categorical variables. Comparisons among different groups were performed by using analysis of variance test. Overall survival was analyzed using the Kaplan–Meier method and differences among groups were assessed by log-rank test. A Cox proportional hazard regression was used to identify independent factors associated with post-resection survival. A two-sided P-value of <0.05 was considered to be statistically significant. All statistical analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC, USA) statistical software package.
Results
General Characteristics of Non-Ampullary–Duodenal Carcinomas and Comparison with both Ampullary Carcinomas and Pancreatic Ductal Adenocarcinomas
There were 29 men and 18 women with non-ampullary–duodenal carcinomas (Table 1). The mean age was 63 years. Common presenting symptoms were anemia (22%), duodenal obstruction (22%), right upper quadrant/epigastric pain (19%), nausea and vomiting (19%), and gastrointestinal bleeding (16%). One patient was incidentally diagnosed when undergoing imaging studies for large gallbladder obstruction. Twenty-eight patients underwent pylorus-sparing pancreaticoduodenectomy (67%), 12 underwent segmental duodenectomy (28%), and 2 underwent classic pancreaticoduodenectomy (5%). The anatomic distribution of the tumor among 39 patients, in which the precise localization of the tumor was properly recorded, was as follows—first segment of duodenum: 3 cases (8%); second (descending) segment of duodenum: 24 cases (62%); third (horizontal) segment of duodenum: 6 cases (15%); fourth (ascending) part of the duodenum: 4 cases (10%); and junction between the third and fourth segments of the duodenum: 2 cases (5%). The mean overall size of tumor was 3.5 cm (vs 2.6 cm for ampullary, P<0.01; and 3.3 cm for pancreatic ductal, P=0.36). The mean invasion size was 2.9 cm (vs 1.9 cm for ampullary, P<0.01; and 3.3 cm for pancreatic ductal, P=0.13). The rate of lymph node positivity was 59% (vs 45 % for ampullary, P=0.08; and 77% for pancreatic ductal, P=0.02). The positive margins were present in only two cases (one with positive proximal margin; and the other with positive duodenal radial margin). The rate of margin positivity was similar to that for ampullary carcinomas (5%), but significantly lower than that of pancreatic ductal carcinomas (23%; P<0.01). Frequency of perineural invasion was 48% (vs 34% for ampullary, P=0.08 and 96% of pancreatic ductal, P<0.01). Frequency of vascular invasion was 76% (vs 63% for ampullary, P=0.11 and 80% for pancreatic ductal, P=0.54). Among 38 patients with adequate information available, only two patients (5%) had documented neoadjuvant chemotherapy and/or radiation before surgical resection.
Comparison of Non-Ampullary–Duodenal Carcinomas with Ampullary–Duodenal Subset of Ampullary Carcinomas
There was no statistically significant difference in age or gender between non-ampullary–duodenal carcinomas and the ampullary–duodenal subset of ampullary carcinomas (Table 2). The mean overall size of non-ampullary–duodenal carcinomas was significantly smaller than that of ampullary–duodenal carcinomas (3.5 cm vs 4.8 cm, P<0.01); however, due to the paucity of adenoma component in non-ampullary–duodenal carcinomas, the mean invasion size was very similar between the two groups (2.9 cm for non-ampullary–duodenal carcinomas vs 3.2 cm for ampullary–duodenal carcinomas, P=0.48). The rate of lymph node positivity was 59% in non-ampullary–duodenal carcinomas vs 64% in ampullary–duodenal carcinoma (P=0.66). There was no significant difference in perineural and vascular invasion rates between the two groups (41% and 69% for ampullary–duodenal cancers vs 48% and 76% for non-ampullary–duodenal carcinomas, respectively; P-values of 0.59 and 0.53, respectively).
Gross Features of Non-Ampullary–Duodenal Carcinomas
Three growth patterns of non-ampullary–duodenal carcinomas were noted: a well-defined plaque-like pattern in 16 cases (Figure 1a), an ulcero-plaque pattern in 16 cases (Figure 1b), and a polypoid-vegetating growth pattern in 6 cases. In contrast, the vast majority of ampullary–duodenal subset of ampullary carcinomas had polypoid-vegetating growth (prominent adenoma component).
Histologic Patterns
By morphologic assessment using the conventional histopathologic criteria, 37 cases (78%) with predominant tubular (glandular) pattern were classified as tubular type. Among these cases, pure intestinal type was only seen in 7 (19%) (Figure 2a) with the remaining 30 (81%) categorized as non-intestinal type. The non-intestinal-type group included gastro-pancreatobiliary pattern in 15 cases (50%) (Figure 2b and c), and hybrid or mixed features in 15 cases (50%; Figure 2d). Atypical and uncharacterized patterns were also identified, that are seldom (if at all) seen elsewhere in the gastrointestinal tract. In particular, two cases (5%) showed a cribriform pattern (Figure 3a). Three cases (6%) focally showed a microcystic/vacuolated pattern (Figure 3b) similar to that recently described in pancreatic adenocarcinoma by Dursun et al.13 Another case (2%), with an oncocytic papillary pattern, was characterized by distinctive papillary growth pattern, consisting of arborizing papillae lined by cuboidal cells with nuclei having single prominent eccentric nucleoli (Figure 3C). One case (2%) showed an unusual comedocarcinoma-like architecture (Figure 3d), mimicking comedo-type in situ carcinoma of the breast. One case (2%) had a superficial ‘goblet-cell-carcinoid’-like component (Figure 3e), characterized by tight clusters of tumor cells with goblet cell or signet ring-like morphology, but was not immunoreactive for neuroendocrine markers and the deeper part of the tumor was mucinous type with signet ring cells floating within the abundant extracellularmucin.
Other rare carcinoma types also occurred, including adenosquamous carcinoma (one case), mucinous (one case), medullary (two cases), and poorly cohesive cell type (two cases). A mucinous component was also identified in four additional cases, but falling short of the criteria for mucinous carcinomas. Besides the adenosquamous case, focal squamoid features were observed in four additional cases.
Only 17 (37%) of non-ampullary–duodenal carcinomas showed an identifiable adenomatous component, and even these were often small or limited in amount.
Changes in the Background Mucosa
In 15 of non-ampullary–duodenal carcinoma cases, the background mucosa showed abnormalities of gastric type or proliferation of Brunner glands. Most of these occurred in those arising in proximal duodenum. Among the 27 cases of non-ampullary–duodenal carcinomas arising in first/second portion of duodenum, gastric heterotopia was found in one case in the proximity with the tumor; foveolar dysplasia, in two cases; surface foveolar metaplasia/Brunner’s gland hyperplasia with/without cystic changes in nine cases; and intestinal adenoma was found only in one case. Among the 12 cases of non-ampullary–duodenal carcinomas arising in third/fourth portion of duodenum, intestinal adenoma was present in nine cases; only one case had both intestinal adenoma and pyloric gland adenoma. No gastric heterotopia, foveolar dysplasia, or any obvious foveolar metaplasia/Brunner’s gland hyperplasia were identified in this group arising in distal duodenum.
Interestingly, in eight cases (17%), the uninvolved mucosa away from carcinoma showed atrophic duodenitis (villous blunting and significantly increased intraepithelial lymphocytes—features characteristic of celiac or similar diseases). In this group, the overall mean age was 63 years with a male to female ratio of 0.6. The mean overall tumor size was 3.5 cm and the mean invasion size was 3.5 cm. All margins were negative. Rates of perineural and lymphovascular invasion were 50% and 75%, respectively, and 50% of cases had positive lymph nodes. The plaque-like growth pattern was observed in four of these, ulcero-plaque growth pattern in three, and polypoid inverted growth pattern in one. The histologic types were heterogeneous, with glandular pattern in five cases (gastro-pancreatobiliary pattern in one case, pure intestinal pattern in one, and mixed pattern in three); medullary in one case, adenosquamous carcinoma in one, and poorly cohesive adenocarcinoma in another. Four of eight patients died at 4–81 months.
Immunoprofile of Non-Ampullary–Duodenal Carcinomas
Immunohistochemical markers that are typically expressed consistently in intestinal neoplasms were relatively low in frequency in non-ampullary–duodenal carcinomas (MUC2 36, CK20 42, and CDX2 44%), whereas those of gastro-pancreatobiliary appeared to be fairly common (CK7 55, MUC1 50, MUC5AC 50, and MUC6 34%). Intestinal-type carcinomas (by morphology) expressed CK7 in 14% of cases and the pancreatobiliary marker MUC1 in 29% (Figure 4a). Notably, gastro-pancreatobiliary tumors (by morphology) expressed MUC1, MUC5AC and MUC6 in 67%, 60% and 67% of the cases, respectively; however, 20% of these tumors expressed the intestinal marker CDX2 (Figure 4b). Most importantly, akin to the ampullary carcinoma, there was often a discrepancy between the phenotype of the preinvasive (adenomatous) and invasive components. In six cases, the adenomatous component showed an intestinal phenotype by morphology and expressed intestinal markers MUC2 and CDX2, with focal, if any, immunoreactivity for either gastric or pancreatobiliary markers, although the invasive adenocarcinoma of these cases was of gastro-pancreatobiliary type by histology and immunoprofile (Figure 5).
Unusual/mixed lineage patterns of non-ampullary–duodenal carcinomas: (a) hematoxylin and eosin (left): intestinal-appearing morphology; and immunohistochemical stains: positive for CK7 (middle) and MUC1 (right) markers. (b) Hematoxylin and eosin (left): pancreatobiliary-appearing morphology; and immunohistochemical stains (right): positive for CDX2.
Recently Ang et al.12 proposed immunohistochemical criteria to subtype the ampullary adenocarcinoma into intestinal, pancreatobiliary, and ambiguous subgroups. By extra-polating their criteria to non-ampullary–duodenal carcinoma, among all 38 non-ampullary–duodenal carcinoma cases with available tissue blocks or unstained slides, 66% of carcinomas were classified as non-intestinal type (18% qualifying as pancreatobiliary, 48% falling into ambiguous type; Table 3). Seven morphologically intestinal cases were all classified as intestinal by Ang’s immunohistochemical criteria. However, 15 morphologically pancreatobiliary cases were subdivided into three categories using Ang’s IHC criteria: intestinal (one case), pancreatobiliary (seven cases), and ambiguous (seven cases). Seventy-seven percentage of the mixed morphologic pattern tumors remained in the same group, whereas 23% were re-classified into intestinal subtype by Ang criteria. Table 4 documents the expression of the different immunohistochemical markers in 38 non-ampullary–duodenal carcinomas, along with their distribution among the histologic subtypes.
DNA Mismatch Repair Protein Deficiency in Non-Ampullary–Duodenal Carcinomas
DNA mismatch repair protein deficiency was detected by immunohistochemistry in six non-ampullary–duodenal carcinomas (13%): two cases showed the loss of MSH2/MSH6 proteins; two showed loss of MSH6 protein; one showed loss of MLH1/PMS2; and one showed loss of MSH1, PMS2, and MSH6 proteins. Unfortunately, a revisit of the clinical histories of these patients from the charts did not reveal any relevant information regarding Lynch syndrome. Of the six cases with DNA mismatch repair protein deficiency, three cases were of the intestinal type and moderately differentiated; the other three cases were of medullary histologic features. Tumor-infiltrating lymphocytes were noted in all six cases, ranging from 2 to 77/HPF. All the cases showed pushing-border infiltration microscopically (Figure 6) and plaque-like growth pattern (Figure 1a), which accounted for 37.5% of the plaque-like growth pattern cases. Furthermore, if plaque-like growth pattern was present, the frequency of mismatch repair protein deficiency was higher (37.5%) than if plaque-like growth was absent (9.1%). This difference was statistically significant (P=0.041). In univariate analysis, these DNA mismatch repair protein deficiency cases seemed to display a more favorable outcome, but the difference in survival did not reach statistical significance (P=0.109).
Transmural lymphoid hyperplasia (Crohn’s like infiltrates) was seen in six patients with one case showing loss of MLH1 and PMS2 protein expression with wild-type BRAF gene, suggesting microsatellite instability. Morphologically, this tumor showed medullary type growth pattern with uninvolved duodenal mucosa showing celiac disease-like features.
Prognosis of Non-Ampullary–Duodenal Carcinomas, and Comparison with Ampullary Carcinomas and Pancreatic Ductal Adenocarcinomas
Of the 30 non-ampullary–duodenal carcinomas with follow-up information available survival ranged from 1–106 months (mean, 18 months). The presence of morphologic gastro-pancreatobiliary pattern, regardless of its extent, appeared to be associated with more aggressive behavior than those that did not, but this did not reach statistical value (P=0.07; Figure 7). None of the other histologic parameters reached statistical significance.
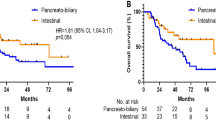
The 3- and 5-year survival rates of resected non-ampullary–duodenal carcinomas were 57% and 57%, respectively, which was similar to those of patients with ampullary carcinomas (62 and 49%; P=0.75), especially, the ampullary–duodenal subset of ampullary carcinomas (59% and 52%, respectively) (P=0.78; Table 2); however, when compared with the ampullary ductal subset of ampullary carcinomas (41% and 29%, respectively), they had much better survival (P<0.001).3 Non-ampullary–duodenal tumors were also associated with incomparably better outcome than pancreatic ductal adenocarcinoma patients (3- and 5-year survival rates of 28% and 18%, respectively; P<0.001; Table 1).
The survival of non-ampullary–duodenal carcinomas with pure or almost exclusively composed of gastro-pancreatobiliary type seemed to have a prognosis similar to the ampullary ductal subtype of ampullary carcinomas and pancreatic ductal carcinomas, at least during the early follow-up period (1-year rate 76% vs 81% vs 65%, respectively). Although its 5-year survival (54%) appeared to be better than ampullary ductal ampullary carcinomas and pancreatic ductal adenocarcinomas (36% vs 18%, respectively), this did not reach statistical significance, perhaps due to the low number of cases (P-values of 0.82 and 0.1; Table 5).
Discussion
In this study, non-ampullary–duodenal carcinomas were found to have several distinctive characteristics. First, unlike other intestinal carcinomas, non-ampullary–duodenal carcinomas less commonly arise from overt adenomatous epithelial lesions. Only 37% of the cases in this study had an identifiable adenoma component, and this was prominent in only 14% of cases, as opposed to lower-intestinal cancers and the ampullary–duodenal subset of ampullary carcinomas, where the vast majority has a prominent adenoma component. Interestingly, the distal duodenal carcinomas were more commonly associated with intestinal-type adenomas (9/12) vs the proximal examples (first and second portion of duodenum) more commonly associated with gastric/Brunner gland abnormalities in the background mucosa (12/27), but seldom with intestinal-type adenoma (only one case).
It is now being increasingly appreciated that carcinomas arising from adenomas are biologically different. Non-ampullary–duodenal carcinomas instead often form plaque/ulcero-plaque-like lesions, which may be partly responsible for their more subtle appearance (and smaller overall size) at diagnosis, compared with the ampullary–duodenal subset of ampullary carcinomas although the size of the invasive components of these two groups is comparable. Of note, this plaque-like growth had a close association with DNA mismatch repair protein deficiency, which we found in 13% of non-ampullary–duodenal carcinomas overall, with all of these showing plaque-like growth pattern, pushing-border infiltration, and tumor-infiltrating lymphocytes microscopically. Considering that DNA mismatch repair protein testing is now performed routinely in colorectal cancer, where the frequency of mismatch repair protein deficiency is also about the same rate,14 a DNA mismatch repair protein testing may have to be considered in at least the cases with these findings, if not all non-ampullary–duodenal carcinomas. The lack of overt adenoma component in most non-ampullary–duodenal carcinomas (along with other findings discussed below) also leads to the impression that they may be arising from Brunner glands (or non-intestinal cell types that are not prone to form adenomas).
In addition, marked contrast with lower-intestinal cancers, most of which are pure intestinal type, non-ampullary–duodenal carcinomas exhibit a striking degree of morphologic versatility. In our study, the gastro-pancreatobiliary and mixed patterns were predominant, with only 19 % of cases qualifying as pure intestinal by morphology and 34% immunohistochemically by Ang criteria proposed for classifying ampullary carcinomas into pancreatobiliary versus intestinal recently.12 Ushike et al.10 noted in their recent study of 38 ‘extra-ampullary–duodenal carcinomas’ that only about a third of their cases qualified as ‘intestinal’, and 50% as gastric, with additional 5% as pancreatobiliary. It should be noted here that our series is predominantly composed of cases from the second segment of the duodenum with only three cases (7%) from first segment, presumably due to our group’s interest in pancreatobiliary tract; whereas, in the study by Ushiku and Lauwers, 40% of their cases were from the first segment, possibly representing the gastric pathologists’ perspective. Nonetheless, both our studies are in accordance that non-ampullary–duodenal carcinomas are more commonly of gastro-pancreatobiliary type.
Non-ampullary–duodenal carcinomas also differ significantly from other intestinal cancers by their immunoprofile15, 16 as well, in that <50% express MUC2, CDX2, and CK20, markers that are consistently positive in lower-intestinal cancers. Instead, gastro-pancreatobiliary markers, which are seldom expressed in intestinal cancers, are fairly common in non-ampullary–duodenal carcinoma (MUC1 50, MUC5AC 50, MUC6 34, and CK7 55%), highlighting this tumor’s closer relationship to gastric/pancreatobiliary (foregut) carcinomas, rather than lower-intestinal ones.
It should also be noted that in a manner similar to ampullary carcinomas, but different than other intestinal carcinomas, non-ampullary–duodenal carcinomas, often show mixed/hybrid phenotypes presumably related to the fact that a variety of cell types reside in this region (including Brunner glands, as well as metaplastic/heterotopic epithelium of gastric and pancreatic origin) that can also occur frequently in the duodenum, and may be responsible for the not uncommon expression of gastro-pancreatobiliary lineage markers (MUC5AC/MUC6/CK7) even in intestinal-appearing cases. As a consequence, it is not surprising that non-ampullary–duodenal carcinomas can give rise to hitherto unrecognized adenocarcinoma patterns (outside the realm of conventional intestinal and pancreatobiliary adenocarcinomas), including comedocarcinoma-like, papillary-oncocytoid, and cribriform, and partially also microcystic although a version of the latter also occurs in pancreatobiliary adenocarcinomas.
Separately, the fact that celiac disease-like changes (atrophy and intraepithelial lymphocytosis) were seen in the duodenal mucosa away from the adenocarcinoma in 17% of the non-ampullary–duodenal carcinomas in our cohort begs the question of whether tumor development may, at least in part, result from an injury mechanism specific to the duodenum. Whether the cases arising in this setting connote different biology or not requires further studies.
In our cohort the 5-year survival rate for non-ampullary–duodenal carcinomas was 57%. This figure is similar to the studies that focused on well-characterized cases of non-ampullary–duodenal carcinomas, where it is ~55% with a range of 54–60% in the largest institutional series on this topic.5, 10, 17, 18 We found that the presence of gastric-pancreatobiliary histology may be associated with more aggressive behavior (although this did not reach statistical significance), which was also reported by others10, 16 and thus it is important to attempt to recognize and report this lineage (no matter the quantity) in any non-ampullary–duodenal carcinoma case.
When compared with a well-characterized cohort of ampullary carcinomas, the prognosis of non-ampullary–duodenal carcinomas is fairly similar, when all ampullary carcinomas are considered together. Not surprisingly, it is the ampullary–duodenal subset of ampullary carcinomas that non-ampullary–duodenal carcinomas are most similar to, whereas the ampullary ductal subset of ampullary carcinoma has a worse outcome than non-ampullary–duodenal carcinoma (5 year, 29% vs 57%). More importantly, the prognosis of non-ampullary–duodenal carcinoma is incomparably better than that of pancreatic ductal adenocarcinoma. Having said that, non-ampullary–duodenal carcinomas with pure or almost exclusively gastro-pancreatobiliary type appear to have aggressiveness similar to the ampullary ductal subset of ampullary carcinomas and pancreatic ductal adenocarcinomas at least at the early phase (1-year rate 76%, 81%, and 65%, respectively); although their long-term survival may prove to be better (54% vs 36% vs 18%, respectively, in this study, which did not reach statistical significance, probably due to low numbers). This is important, because, for non-ampullary–duodenal carcinoma that occurs close to the pancreas, pancreatic ductal adenocarcinoma with secondary invasion into duodenum becomes an important and highly challenging differential. These findings also highlight the importance of accurate grossing in pancreatoduodenectomy specimens in identifying the site of origin of these tumors, as our study shows that non-ampullary–duodenal carcinomas have much better outcomes than their pancreatic ductal carcinoma and ampullary ductal ampullary carcinoma counterparts. These findings are in sharp contrast to those of Westgaard et al.,19 who in their studies of all periampullary adenocarcinomas (non-ampullary–duodenal carcinomas included) suggested that it is histology (pancreatobiliary vs intestinal) and not location that played a critical role in the prognosis of these tumors. It should also be kept in mind that besides behavioral differences there may be various etiopathogenetic and molecular mechanistic differences between the cancers arising from different areas of this relatively small region, and each may require different management approaches in the future accordingly.
In conclusion, this study elucidates that non-ampullary–duodenal cancers have clinicopathologic characteristics similar to ampullary–duodenal cancers, but different from the other intestinal and pancreatobiliary tract cancers. Compared with lower-intestinal cancers non-ampullary–duodenal carcinomas often exhibit gastro-pancreatobiliary lineage, and are seldom of pure intestinal type, a fact that should be considered when devising site-specific treatment protocols for these tumors. Non-ampullary–duodenal carcinomas also appear to have different carcinogenic mechanisms, often skipping the adenoma-carcinoma sequence (only a third has identifiable adenoma component). This, combined with frequent plaque-like growth (which seems to have an association with DNA mismatch repair protein deficiency), warrants further analysis for developing more specific therapies. It is also important for pathologists to recognize the morphologic versatility of these carcinomas for accurate diagnosis that may in part be related to the epithelial diversity of this region. The paucity of adenomatous elements, common plaque-like growth, and tendency for gastro-pancreatobiliary lineage with hitherto unrecognized patterns discovered in this study suggest that a subset of non-ampullary–duodenal carcinomas may be arising from submucosal ductular/glandular elements (including Brunner glands) or heterotopic tissue that distinguishes this region from other segments of the gastrointestinal tract.
References
Struck A, Howard T, Chiorean EG et al. Non-ampullary duodenal adenocarcinoma: factors important for relapse and survival. J Surg Oncol 2009;100:144–148.
Yeo CJ, Sohn TA, Cameron JL, et al. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg 1998;227:821–831.
Adsay V, Ohike N, Tajiri T et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol 2012;36:1592–1608.
Onkendi EO, Boostrom SY, Sarr MG, et al. 15-year experience with surgical treatment of duodenal carcinoma: a comparison of periampullary and extra-ampullary duodenal carcinomas. J Gastrointest Surg 2012;16:682–691.
Zenali M, Overman MJ, Rashid A et al. Clinicopathologic features and prognosis of duodenal adenocarcinoma and comparison with ampullary and pancreatic ductal adenocarcinoma. Hum Pathol 2013;44:2792–2798.
Washington K, Berlin J, Branton P et al. Protocol for the Examination of Specimens from Patients with Carcinoma of the Ampulla of Vater. The College of American Pathologist, 2016.
Edge SB American Joint Committee on Cancer AJCC Cancer Staging Manual, 7th edn. Springer: New York, USA, 2010, pp xiv, 648.
Reid MD, Balci S, Ohike N et al. Ampullary carcinoma is often of mixed or hybrid histologic type: an analysis of reproducibility and clinical relevance of classification as pancreatobiliary versus intestinal in 232 cases. Mod Pathol 2016 [e-pub ahead of print].
Ohike N, Kim GE, Tajiri T, et al. Intra-ampullary papillary-tubular neoplasm (IAPN): characterization of tumoral intraepithelial neoplasia occurring within the ampulla: a clinicopathologic analysis of 82 cases. Am J Surg Pathol 2010;34:1731–1748.
Ushiku T, Arnason T, Fukayama M, et al. Extra-ampullary duodenal adenocarcinoma. Am J Surg Pathol 2014;38:1484–1493.
Bosman FT . World Health Organization, International Agency for Research on Cancer WHO classification of tumours of the digestive system, 4th edn. International Agency for Research on Cancer: Lyon, France, 2010, 417 pp.
Ang DC, Shia J, Tang LH et al. The utility of immunohistochemistry in subtyping adenocarcinoma of the ampulla of vater. Am J Surg Pathol 2014;38:1371–1379.
Dursun N, Feng J, Basturk O, et al. Vacuolated cell pattern of pancreatobiliary adenocarcinoma: a clinicopathological analysis of 24 cases of a poorly recognized distinctive morphologic variant important in the differential diagnosis. Virchows Arch 2010;457:643–649.
Ashktorab H, Ahuja S, Kannan L et al. A meta-analysis of MSI frequency and race in colorectal cancer. Oncotarget 2016;7:34546–34557.
Overman MJ, Pozadzides J, Kopetz S, et al. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer 2010;102:144–150.
Jun SY, Eom DW, Park H et al. Prognostic significance of CDX2 and mucin expression in small intestinal adenocarcinoma. Mod Pathol 2014;27:1364–1374.
Barnes G Jr., Romero L, Hess KR, et al. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol 1994;1:73–78.
Rose DM, Hochwald SN, Klimstra DS, et al. Primary duodenal adenocarcinoma: a ten-year experience with 79 patients. J Am Coll Surg 1996;183:89–96.
Westgaard A, Pomianowska E, Clausen OP, et al. Intestinal-type and pancreatobiliary-type adenocarcinomas: how does ampullary carcinoma differ from other periampullary malignancies? Ann Surg Oncol 2013;20:430–439.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Xue, Y., Vanoli, A., Balci, S. et al. Non-ampullary–duodenal carcinomas: clinicopathologic analysis of 47 cases and comparison with ampullary and pancreatic adenocarcinomas. Mod Pathol 30, 255–266 (2017). https://doi.org/10.1038/modpathol.2016.174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.174
This article is cited by
-
A case of non-ampullary duodenal adenosquamous carcinoma with successful emergency pancreaticoduodenectomy for gastrointestinal hemorrhage
Surgical Case Reports (2023)
-
Claudin-18 expression in small bowel adenocarcinoma: a clinico-pathologic study
Virchows Archiv (2022)
-
APC mutations are common in adenomas but infrequent in adenocarcinomas of the non-ampullary duodenum
Journal of Gastroenterology (2021)
-
Small bowel carcinomas in celiac or Crohn's disease: distinctive histophenotypic, molecular and histogenetic patterns
Modern Pathology (2017)