Abstract
Various histological variants of papillary thyroid carcinoma have been reported, some with clinical implications, some with peculiar, sometimes misleading morphologies. One of these rare and poorly characterized variants is papillary thyroid carcinoma with nodular fasciitis-like stroma, of which fewer than 30 cases have been documented, mostly as isolated reports. It is a dual tumor comprising a malignant epithelial proliferation that harbors typical features of conventional papillary thyroid carcinoma, admixed with a prominent mesenchymal proliferation resembling nodular fasciitis or fibromatosis. Thus, the terms papillary thyroid carcinoma with nodular fasciitis-like stroma and papillary thyroid carcinoma with fibromatosis-like stroma are used interchangeably; however, the former term suggests a self-limited and regressing disease, whereas the latter one suggests a recurrent and potentially aggressive one. Better genetic and ultrastructural characterization could lead to more appropriate terminology and management. We performed detailed clinicopathological and molecular analyses of two cases of PTC with prominent mesenchymal proliferation that developed in the thyroid gland of two male patients aged 34 and 48. In both cases, the epithelial component harbored a heterozygous somatic activating BRAF mutation (p.V600E). Also, in both cases, the mesenchymal component showed typical aberrant nuclear and cytoplasmic immunoreactivity for β-catenin and harbored a heterozygous somatic activating mutation in the corresponding CTNNB1 gene (p.S45P). This mutation has never been reported in thyroid stroma; in other tissues, it is typical of desmoid-type fibromatosis rather than nodular fasciitis-like stroma. We therefore propose that in cases of papillary thyroid carcinoma with a prominent mesenchymal component, mutations in CTNNB1 should be sought; when they are present, the term 'papillary thyroid carcinoma with desmoid-type fibromatosis' should be used. As the mesenchymal component of these tumors is not expected to concentrate radioactive iodine, special considerations apply to clinical evaluation and follow-up, which should be brought to the attention of the treating specialist.
Similar content being viewed by others
Main
The most common histological subtype of thyroid carcinoma is papillary thyroid carcinoma, representing up to 80% of all well-differentiated forms.1 The global incidence of papillary thyroid carcinoma has been increasing over the last decades, but its prognosis remains overall favorable, with a stable mortality rate for its more common variants, that is the classical and follicular variants.2, 3 Management includes surgery often followed by radioactive iodine.
Different histological variants of papillary thyroid carcinoma have been described, some with clinical implications, some challenging to recognize because of their rarity and/or peculiar morphology. One of these rare and poorly characterized variants is papillary thyroid carcinoma with exuberant mesenchymal proliferation.2 It is characterized by a double component: a malignant epithelial proliferation harboring the typical features of conventional papillary thyroid carcinoma (overlapping ground-glass and grooved nuclei with pseudoinclusions) and a prominent mesenchymal cell component resembling nodular fasciitis or fibromatosis, hence the denominations papillary thyroid carcinoma with nodular fasciitis-like stroma and PTC with fibromatosis-like stroma. According to the World Health Organization (WHO) Classification of Tumors of Endocrine Organs, these two histological subtypes of papillary thyroid carcinoma are synonyms.2 The great majority of cases described contain >80% of mesenchymal cells and <20% of malignant follicular cells. The bulk of the tumor is thus represented by mesenchymal cells, usually fibroblasts and myofibroblasts. Several studies showed an aberrant immunohistochemical expression of β-catenin in the nucleus and cytoplasm of mesenchymal cells, but the molecular cause for the activation of the Wnt signaling pathway in these tumors remains unknown.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Aberrant nuclear accumulation of β-catenin is reported in different neoplastic and reactive conditions and is related to direct or indirect alterations of the Wnt/β-catenin pathway.19 Among thyroid carcinomas, cytoplasmic and nuclear accumulation of β-catenin has been reported only in the cribriform-morular variant, which occurs sporadically or as an extra-intestinal manifestation of the familial adenomatous polyposis syndrome; in this case, it is associated with the presence in the follicular cells of activating somatic mutations in CTNNB1, the gene encoding β-catenin.20 More commonly, 40–50% of thyroid carcinomas present heterozygous somatic mutations in BRAF, the most frequent being the p.V600E mutation (amino-acid substitution of glutamic acid for valine at position 600).21
We report here two cases of papillary thyroid carcinoma associated with a prominent mesenchymal proliferation characterized by a BRAF V600E mutation in the epithelial component and by an activating CTNNB1 mutation in the mesenchymal component. Based on the presence of the CTNNB1 mutation and supported by the ultrastructural demonstration of collagen fibers, we propose that a more suitable terminology for this neoplasm is 'papillary thyroid carcinoma with desmoid-type fibromatosis'.
Materials and methods
Patient Selection
A 34-year-old man with unremarkable personal and familial medical history presented with a neck mass that he had noticed growing for 1 year. Physical examination revealed a firm nodule in the right thyroid lobe, mobile while swallowing, without associated pain, dysphonia or dysphagia. Thyroid function tests were normal. Ultrasound-guided thyroid fine-needle aspiration revealed a 20 mm thyroid nodule considered benign in a private clinic (slides not available for review). Six months later, the patient presented to the Lausanne University Hospital because of further enlargement of the thyroid nodule. Thyroid ultrasound revealed a partly cystic 38 mm nodule with rare microcalcifications (Figure 1a). The successive ultrasound-guided fine-needle aspiration rendered a diagnosis of atypia of undetermined significance/follicular lesion of undetermined significance, according to the Bethesda system for reporting thyroid cytology.22 The rapid increase of the tumor size prompted immediate right lobo-isthmectomy rather than a repeat fine-needle aspiration in 3–6 months as suggested by the Bethesda system for reporting thyroid cytology. After identification of a papillary thyroid carcinoma on pathology, completion thyroidectomy (without lymphadenectomy) was performed, followed by radioactive iodine ablation treatment (30 mCi using recombinant TSH stimulation).
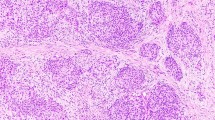
Ultrasound aspect and cyto-histological findings. (a) Case #1. Ultrasound examination showed a large thyroid nodule (38 × 36 × 30 mm) in the right thyroid lobe, with sparse microcalcifications and a central hyperechoic zone. (b) Case #1. Fine-needle aspiration showed aggregates of follicular cells in sheet and pseudo-papillary structures with enlarged and elongated nuclei, grooves and focally clearing chromatin (inset). All cells showed granular and oxyphilic cytoplasm (smear, Papanicolaou staining, 400 ×). (c) Case #1. On liquid-based cytology, fragmented spindle cells with fusiform nuclei corresponding to fibroblasts/myofibroblasts were seen in a myxoid background (liquid-based cytology, Papanicolaou staining, 400 ×). (d) Case #1. On gross examination, the right lobe measured 85 × 60 × 45 mm and almost entirely comprised a whitish, solid, well-circumscribed nodule that had increased rapidly in size in comparison with the last ultrasound examination (a). (e) Case #1. The tumor consisted of two distinct components: stromal and epithelial (arrows). The abundant stromal component corresponded to 95% of the entire tumor, whereas the epithelial component to the remaining 5%. Scan of the slide (hematoxylin–eosin). (f) Case #1. Microscopically, the stroma was composed by spindled-shaped cells arranged in interlacing fascicles accompanied by thick collagen fibers (left part), whereas the epithelial component (right part) comprised small foci of oncocytic papillary thyroid carcinoma, arranged in papillae and solid nests (hematoxylin–eosin, 200 ×). Inset shows a nuclear pseudo-inclusion (arrow, hematoxylin–eosin, 400 ×). (g) Case #2. The tumoral stromal component corresponded to 85% of the entire tumor and the epithelial component (arrows) to the remaining 15%. Scan of the slide (hematoxylin–eosin). (h) Case #2. Microscopically, the stromal and epithelial components were similar to case #1, with the only difference that non-necrotizing granulomas (sarcoid-like, arrow) were found inside the epithelial oxyphilic component (hematoxylin–eosin, 400 ×).
A 48-year-old euthyroid man was referred for investigation of a right thyroid lobe nodule to the Arcispedale Santa Maria Nuova Hospital. US evaluation showed a 28 mm nodule with ill-defined borders and no microcalcification; there were no cervical lymphadenopathies. Ultrasound-guided fine-needle aspiration yielded a diagnosis of suspicious for papillary thyroid carcinoma according to the Bethesda system for reporting thyroid cytology. In agreement with the patient, total thyroidectomy was performed. The patient was lost to follow-up in the post-operative period.
Cyto-Histopathologic Analyses
Cytology preparations were available for review only for case #1, limited to the second fine-needle aspiration (cytology slides of case #2 were not found in the pathology archives). Aspired material of case #1 (three passes) was smeared onto six slides and stained with Papanicolaou (five slides) and Giemsa (one slide) stains; the needle was also rinsed in CytoLyt (Hologic Inc., Marlborough, MA, USA) and one additional slide was prepared using the ThinPrep protocol (Hologic Inc., Marlborough, MA, USA) for liquid-based cytology.
Histopathology features were assessed for both cases using hematoxylin and eosin-stained sections from formalin-fixed, paraffin-embedded tissue. Representative tissue blocks containing both epithelial and mesenchymal components were selected for immunohistochemical analysis, electron microscopy and molecular studies.
Immunohistochemical Analyses
Immunohistochemical stainings were performed on a Benchmark XT System (Ventana Medical Systems, Tucson, TX, USA) on deparaffinized sections from selected formalin-fixed, paraffin-embedded tissue blocks with the primary antibodies listed in Table 1. Antibody detection and final staining were carried out with the UltraView Universal DAB Detection Kit (Ventana Medical Systems).
Ultrastructural Analyses
Ultrastructural features were assessed using a representative block of formalin-fixed, paraffin-embedded tissue for each case. Samples were post-fixed in 1.3% osmium tetroxide, dehydrated and embedded in an epoxy medium. Blocks were cut using an ultramicrotome (Ultracut E, Reichert-Jung Optische Werke AG, Wien, Austria) and mounted on 3-mm 200-mesh copper grids. Slides were stained with methylene blue, examined and photographed with a Philips CM10 transmission electron microscope combined with a MegaView III Soft Imaging system.
Molecular Analyses
For each case, a formalin-fixed, paraffin-embedded tissue block containing both epithelial and mesenchymal components was selected for molecular analyses. Each component was separately outlined on an hematoxylin–eosin slide, identified on corresponding toluidine blue-stained sections, manually microdissected under a microscope and collected for genomic DNA extraction (Maxwell 16 FFPE Plus LEV DNA Purification kit, Promega, Madison, WI, USA). Genomic DNA was amplified by polymerase chain reaction using custom primers for exon 15 of BRAF and exon 3 of CTNNB1. Amplicons were assessed by capillary electrophoresis (QIAxcel, Qiagen, Hilden, Germany). Mutation analysis was performed by pyrosequencing (PyroMark, Qiagen), spanning codons 599–602 of BRAF, and codons 34–39 and 41–46 of CTNNB1, with a technical sensitivity of approximately 10% mutant allele content. Each assay was performed in duplicate, in parallel with a wild-type DNA control and a no template control. Details about primer sequences and other PCR and sequencing parameters are available upon request.
Results
Cytologic Findings
In case #1, the fine-needle aspiration performed in the Lausanne University Hospital was hypocellular, showing rare aggregates of follicular cells with oncocytic cytoplasm and enlarged nuclei with irregular nuclear membranes and focal chromatin clearing (Figure 1b). These cells were also observed in the liquid-based cytology preparation. As a result of these findings, the diagnosis rendered was atypia of undetermined significance/follicular lesion of undetermined significance. After histological assessment, the cytology slides were reviewed and mesenchymal cells were seen only in the liquid-based cytology preparation as isolated spindle cells in a myxoid substance (Figure 1c).
According to the cytology report (slides were not available for review), fine-needle aspiration findings in case #2 showed a prominent atypical epithelial component with nuclear grooves and some intranuclear pseudoinclusions. These features were considered suspicious but not diagnostic of papillary thyroid carcinoma and a diagnosis of suspicious for papillary thyroid carcinoma was given. The description also included the presence of scattered fragments of mesenchymal tissue, but these were not considered as relevant for the diagnosis.
Histopathological Findings
In case #1, the right lobe measured 85 mm after formalin fixation. On cut section, the entire lobe was replaced by a well-circumscribed but non-encapsulated, firm and homogeneous whitish nodule (Figures 1d and e). In comparison with the latest ultrasound examination that reported a 38 mm nodule, the diameter of the lesion had more than doubled at the time of surgery. Histologically, it consisted of two intermingled but distinct cell populations, one of mesenchymal nature and the other of epithelial nature. The mesenchymal component represented about 95% of the whole tumor, and consisted of monomorphous spindle-shaped cells with elongated nuclei arranged in interlacing fascicles, accompanied by thick collagen fibers (Figures 1e and f) and small lymphocytic aggregates. Spindle cells showed neither cytological atypia nor mitotic figures, but they infiltrated focally through the thyroid capsule into the surrounding skeletal muscle. Surgical resection margins were free of tumor. No necrosis was observed in this component. The epithelial component, representing 5% of the tumor, was arranged in papillae, trabecular structures or small follicles. Malignant epithelial cells showed typical nuclear features of papillary thyroid carcinoma, including pale, ground-glass nuclei with nuclear grooves, occasional nuclear pseudoinclusions and a cytoplasm of variable abundance, sometimes oncocytic (Figure 1f). The remaining thyroid parenchyma showed small foci of chronic thyroiditis.
In case #2, the nodule measured 28 mm in its greatest axis, with a vaguely demarcated border (Figure 1g). It showed the same histological features as in case #1, however, with a more prominent epithelial component (about 15% of the whole tumor) (Figure 1g). Oncocytic change was present. Interestingly, sarcoid-like non-necrotizing granulomas were found in contact with the epithelial component (Figure 1h). Surgical resection margins were free of tumor.
Immunohistochemical Findings
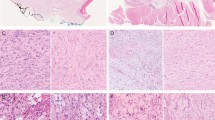
The immunohistochemical findings are summarized in Table 2. The epithelial component of the tumors was positive for pancytokeratin (AE1/AE3), TTF-1, thyroglobulin, galectin-3 and HBME-1, and showed membranous staining for β-catenin (Figure 2a). The mesenchymal component of the tumors showed focal cytoplasmic staining for smooth muscle actin and vimentin, as well as aberrant nuclear and cytoplasmic staining for β-catenin (Figure 2b), but was negative for pancytokeratin (AE1/AE3), desmin, S100 protein, CD34, CD99, Bcl-2, thyroglobulin and CD117.
Immunohistochemical, ultrastructural and molecular findings of case #1. (a) β-Catenin immunohistochemical staining showed diffuse and intense membrane positivity and nuclear negativity in the epithelial component (200 ×). (b) β-Catenin immunohistochemical staining demonstrated diffuse and intense nuclear and cytoplasmic staining in the stromal component. The arrow shows negative endothelial cell nuclei (200 ×). (c and inset) Collagen fibers observed in the proximity of myofibroblastic cells. (d) At electron microscopy, the stromal cells showed abundant rough endoplasmic reticulum. (e) CTNNB1 mutation analysis: the pyrogram revealed wild-type CTNNB1 codon 45 sequence in the papillary thyroid carcinoma component, whereas a heterozygous c.133T>C (p. S45P) mutation was detected in CTNNB1 in the stromal component (the same for case #2). (f) BRAF mutation analysis: pyrosequencing demonstrated a c.1799T>A (p.V600E) mutation in BRAF in the papillary thyroid carcinoma component, whereas codon 600 of this gene was wild-type in the stromal component (the same for case #2).
Ultrastructural Findings of the Mesenchymal Component
Electron microscopy was attempted on tissue retrieved after formalin fixation. Within the limits imposed by suboptimal fixation, collagen fibers and fragments of collagen fibers were observed in the proximity of mesenchymal cells, implying a fibroblastic differentiation at the ultrastructural level (Figure 2c). Abundant rough endoplasmic reticulum was also seen, a typical observation in cells producing large proteins like collagen (Figure 2d).
Molecular Findings
Both components were isolated by microdissection from toluidine blue-stained sections, each of them being composed of >90% of cells of interest. In both cases, DNA sequencing revealed a heterozygous c.133T>C (p.S45P) mutation in exon 3 of CTNNB1 (reference sequence: NM_001904.3), exclusively present in the mesenchymal component, and a heterozygous c.1799T>A (p.V600E) BRAF mutation (reference sequence: NM_004333.4), only detected in the epithelial component (Figures 2e and f).
Discussion
Papillary thyroid carcinoma with nodular fasciitis-like stroma or papillary thyroid carcinoma with fibromatosis-like stroma is a rare variant of papillary thyroid carcinoma, described for the first time by Ostrowski et al23 as papillary thyroid carcinoma with fibromatosis-like stroma and by Chan et al9 as papillary thyroid carcinoma with nodular fasciitis-like stroma.9 Since then, fewer than 30 cases have been reported for both nosological definitions, with an incidence of 0.5% of all papillary thyroid carcinomas in the series by Chan et al9 and 0.17% in the series by Mizukami et al.55, 11 In our two institutions (Lausanne University Hospital, Lausanne, Switzerland and Arcispedale Santa Maria Nuova-IRCCS, Reggio Emilia, Italy), only two cases have been retrieved from archival series, representing 0.03% and 0.04% of all thyroid malignancies and of all papillary thyroid carcinomas, respectively. This difference could be related to the different ethnic backgrounds.
In the reported cases, a diagnosis of papillary thyroid carcinoma with nodular fasciitis-like stroma/papillary thyroid carcinoma with fibromatosis-like stroma has never been made on cytological grounds alone. Either one or both components may be sampled by the fine-needle aspiration, potentially leading to misdiagnosis of the lesion as a variety of reactive, benign or malignant conditions, including the sclerotic variant of papillary thyroid carcinoma, sarcoma or even anaplastic carcinoma. 17, 8 In our cases, the mesenchymal component was not present at all on the smears for patient 1 and was only described but not considered in the final diagnosis for patient 2.
The distinctiveness of this neoplasm as described by the WHO is represented by its dual nature: a prominent mesenchymal cell proliferation resembling nodular fasciitis or fibromatosis—hence the terms papillary thyroid carcinoma with nodular fasciitis-like stroma and papillary thyroid carcinoma with fibromatosis-like stroma, respectively—and a usually minor malignant epithelial proliferation displaying the typical features of conventional papillary thyroid carcinoma.2 Indeed, in our cases the mesenchymal component was predominant (95% and 85% in case 1 and 2, respectively). The mesenchymal cells are apparently myofibroblastic in nature and produce collagen, as confirmed by electron microscopy. The epithelial proliferation shows typical nuclear features of PTC and oncocytic metaplasia.
Histologically, fibromatosis and nodular fasciitis have many common features, notably the cytologically bland spindle cells. Mitotic figures occur in both lesions, but are much less frequent in fibromatosis than in nodular fasciitis.24 The former usually lacks lymphocytic infiltrate and extravasated red blood cells, features often present in nodular fasciitis.13 Despite their morphological similarity, distinction between nodular fasciitis and fibromatosis has important clinical implications: nodular fasciitis is considered a self-limited, spontaneously regressing lesion (« transient neoplasia »), whereas fibromatosis has an aggressive clinical course, often with local recurrences; in consequence, surgery is often more extensive in the case of fibromatosis. In the two cases presented here, both components were completely resected with negative margins. Patient 1 is in complete remission after radioactive iodine treatment. Patient 2 is lost to follow-up.
The pathogenesis of the two distinct components is likely to be different, especially because an unrelated gene mutation was identified in each component. Being neoplastic in nature, the mesenchymal component observed in our cases can be considered neither as a desmoplastic reaction developing in contact with a carcinoma, nor as a metaplastic spindle cell transformation of a follicular-derived lesion such as that found in cases described by Vergilio et al2525, in which mesenchymal cells were positive for thyroglobulin. Other scenarios such as a collision of two independently originating neoplasms are even less likely. Although the exact pathogenesis remains unknown, there are other examples of carcinomas associated with a similar mesenchymal component, mostly described in breast, but more rarely also in lung.26, 27
The Wnt/β-catenin pathway is involved in tumorigenesis in different settings, in neoplastic, metabolic and fibrosing diseases. Among fibrosing diseases, high levels of β-catenin mRNA are found in aggressive deep fibromatosis, a sporadic neoplastic, locally invasive proliferation of fibroblasts originating from musculoaponeurotic planes.28 The somatic mutation of exon 3 of CTNNB1 gene found in the mesenchymal cells of our cases has also been reported in thyroid epithelial cells, mainly in the cribriform-morular variant of papillary thyroid carcinoma.20 This variant occurs in a sporadic form or as an extra-intestinal component of the familial adenomatous polyposis syndrome, of which it may be the initial clinical manifestation.20 In our cases, there were no clinical signs of familial adenomatous polyposis syndrome, and the mutation in exon 3 of CTNNB1 was not present in the follicular malignant cells. In contrast, the presence of the mutation in the myofibroblastic proliferation confirms a tumorigenic alteration of the Wnt/β-catenin pathway similar to the one observed in fibromatosis, but never detected in nodular fasciitis.
BRAF mutations leading to the constitutive activation of the MAPK kinase pathway are leading genetic events in papillary thyroid carcinoma, present in >50% of cases.21, 29 The specificity for malignancy of BRAF mutation is very high (almost 100%), as it has never been described in benign thyroid conditions, which justifies its role as a diagnostic marker in fine-needle aspiration indeterminate specimens.30 Its role as a negative prognostic marker has not been definitively confirmed, but its presence seems to be associated with pathological features of aggressiveness, such as extrathyroidal extension and lymph node metastasis.31, 32, 33 The most common BRAF mutated variant is p.V600E, which is characteristically present in the classical variant, whereas the p.K601E variant is more commonly associated to the follicular variant of papillary thyroid carcinoma.1, 34 The presence of the BRAF mutation is not exclusive to thyroid neoplasia, but is described also in other malignancies, notably in melanoma, lung carcinoma and colon carcinoma. Interestingly, it is also present in 19% of cases of pediatric aggressive fibromatosis, where it is variably associated with CTNNB1 mutations, while is absent in adult aggressive fibromatosis.35
In conclusion, we propose that in cases of PTC with a prominent mesenchymal component, mutations in CTNNB1 should be sought; when they are present, the tumor should be termed 'papillary thyroid carcinoma with desmoid-type fibromatosis' rather than 'PTC with fibromatosis-like stroma' or 'papillary thyroid carcinoma with nodular fasciitis-like stroma'. As the mesenchymal component does not express thyroglobulin, the use of thyroglobulin as a tumor marker is only partly informative for this papillary thyroid carcinoma variant; moreover, because the mesenchymal cells are not expected to concentrate iodine, it is unlikely that radioactive iodine treatment will have an effect on this component, although clinical evidence is still lacking on this issue. Appropriate resection margin status of the mesenchymal component is a critical parameter to assess on histopathology: as Cates et al36 have shown, patients with primary desmoid-type fibromatosis have an increased risk of local recurrence when resection margins are positive or close (tumor clearance <1 mm). These important clinical considerations should be transmitted to the treating clinicians in each case, as they are unlikely to be familiar with this rare tumor.
References
Papp S, Asa SL . When thyroid carcinoma goes bad: a morphological and molecular analysis. Head Neck Pathol 2015; 9: 16–23.
LiVolsi VA, Albores-Saavedra J, Asa SL et al, Papillary carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, (eds);. World Health Organization Classification of Tumours: Pathology and genetics of tumours of endocrine organs, 1st edn. IARC Press: Lyon, France, 2004, pp 127–136.
Davies L, Welch HG . Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014; 140: 317–322.
Terayama K, Toda S, Yonemitsu N et al, Papillary carcinoma of the thyroid with exuberant nodular fasciitis-like stroma. Virchows Arch 1997; 431: 291–295.
Ginter PS, Scognamiglio T . Papillary thyroid carcinoma with nodular fasciitis-like stroma: a usual entity with distinctive morphology. Int J Surg Pathol 2015; 23: 305–307.
Leal II, Carneiro FP, Basílio-de-Oliveira CA et al, Papillary carcinoma with nodular fasciitis-like stroma—a case report in pregnancy. Diagn Cytopathol 2008; 36: 139–141.
Yang YJ, LiVolsi VA, Khurana KK . Papillary thyroid carcinoma with nodular fasciitis-like stroma. Pitfalls in fine-needle aspiration cytology. Arch Pathol Lab Med 1999; 123: 838–841.
Basu S, Nair N, Shet T et al, Papillary thyroid carcinoma with exuberant nodular fasciitis-like stroma: treatment outcome and prognosis. J Laryngol Otol 2006; 120: 338–342.
Chan JK, Carcangiu ML, Rosai J . Papillary carcinoma of thyroid with exuberant nodular fasciitis-like stroma. Report of three cases. Am J Clin Pathol 1991; 95: 309–314.
Toti P, Tanganelli P, Schürfeld K et al, Scarring in papillary carcinoma of the thyroid: report of two new cases with exuberant nodular fasciitis-like stroma. Histopathology 1999; 35: 418–422.
Mizukami Y, Kurumaya H, Kitagawa T et al, Papillary carcinoma of the thyroid gland with fibromatosis-like stroma: a case report and review of the literature. Mod Pathol 1995; 8: 366–370.
Lee M, Song JS, Chun SM et al, Protuberant fibro-osseous lesions of the temporal bone: two additional case reports. Am J Surg Pathol 2014; 38: 1510–1515.
Na KY, Kim HS, Sung JY et al, Papillary carcinoma of the thyroid gland with nodular fasciitis-like stroma. Korean J Pathol 2013; 47: 167–171.
Wu Z, Chu X, Fan S et al, Papillary thyroid carcinoma with fibromatosis-like stroma: a case report and review of the literature. Oncol Lett 2013; 5: 215–217.
Lee YS, Nam KH, Hong SW et al, Papillary thyroid carcinoma with nodular fasciitis-like stroma. Thyroid 2008; 18: 577–578.
Inaba M, Umemura S, Satoh H et al, Papillary thyroid carcinoma with fibromatosis-like stroma: a report of two cases. Endocr Pathol 2002; 13: 219–225.
Us-Krasovec M, Golouh R . Papillary thyroid carcinoma with exuberant nodular fasciitis-like stroma in a fine needle aspirate. A case report. Acta Cytol 1999; 43: 1101–1104.
Michal M, Chlumska A, Fakan F . Papillary carcinoma of thyroid with exuberant nodular fasciitis-like stroma. Histopathology 1992; 21: 577–579.
Enzo MV, Rastrelli M, Rossi CR et al, The Wnt/β-catenin pathway in human fibrotic-like diseases and its eligibility as a therapeutic target. Mol Cell Ther 2015; 30: 1.
Xu B, Yoshimoto K, Miyauchi A et al, Cribriform-morular variant of papillary thyroid carcinoma: a pathological and molecular genetic study with evidence of frequent somatic mutations in exon 3 of the beta-catenin gene. J Pathol 2003; 199: 58–67.
Guerra A, Sapio MR, Marotta V et al, The primary occurrence of BRAF(V600E) is a rare clonal event in papillary thyroid carcinoma. J Clin Endocrinol Metab 2012; 97: 517–524.
Cibas ES, Ali SZ . The Bethesda system for reporting thyroid cytopathology. Thyroid 2009; 19: 1159–1165.
Ostrowski MA, Moffat FL, Asa SL et al, Myxomatous change in papillary carcinoma of thyroid. Surg pathol 1989; 2: 249–256.
Goldstein JA, Cates JM . Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol 2015; 22: 260–266.
Vergilio J, Baloch ZW, LiVolsi VA . Spindle cell metaplasia of the thyroid arising in association with papillary carcinoma and follicular adenoma. Am J Clin Pathol 2002; 117: 199–204.
Gobbi H, Simpson JF, Borowsky A et al, Metaplastic breast tumors with a dominant fibromatosis-like phenotype have a high risk of local recurrence. Cancer 1999; 85: 2170–2182.
Tajima S, Takanashi Y, Koda K . Squamous cell carcinoma of the lung with highly proliferating fibromatosis-like stroma: a rare phenomenon. Int J Clin Exp Pathol 2015; 8: 5870–5876.
Saito T, Oda Y, Kawaguchi K et al, Possible association between higher beta-catenin mRNA expression and mutated beta-catenin in sporadic desmoid tumors: real-time semiquantitative assay by TaqMan polymerase chain reaction. Lab Invest 2002; 82: 97–103.
Kondo T, Ezzat S, Asa SL . Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 2006; 6: 292–306.
Su X, Jiang X, Xu X et al, Diagnostic value of BRAF (V600E)-mutation analysis in fine-needle aspiration of thyroid nodules: a meta-analysis. Onco Targets Ther 2016; 9: 2495–2509.
Xing M, Alzahrani AS, Carson KA et al, Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013; 309: 1493–1501.
Gouveia C, Can NT, Bostrom A et al, Lack of association of BRAF mutation with negative prognostic indicators in papillary thyroid carcinoma: the University of California, San Francisco, experience. JAMA Otolaryngol Head Neck Surg 2013; 139: 1164–1170.
Gandolfi G, Sancisi V, Piana S et al, Time to re-consider the meaning of BRAF V600E mutation in papillary thyroid carcinoma. Int J Cancer 2015; 137: 1001–1011.
Cancer Genome Atlas Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159: 676–690.
Meazza C, Belfiore A, Busico A et al, AKT1 and BRAF mutations in pediatric aggressive fibromatosis. Cancer Med 2016; 5: 1204–1213.
Cates JMM, Stricker TP . Surgical resection margins in desmoids-type fibromatosis. A critical reassessment. Am J Surg Pathol 2014; 38: 1707–1714.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rebecchini, C., Nobile, A., Piana, S. et al. Papillary thyroid carcinoma with nodular fasciitis-like stroma and β-catenin mutations should be renamed papillary thyroid carcinoma with desmoid-type fibromatosis. Mod Pathol 30, 236–245 (2017). https://doi.org/10.1038/modpathol.2016.173
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.173
This article is cited by
-
Papillary thyroid carcinoma with fibromatosis-like stroma: a case report and review of the literature
BMC Endocrine Disorders (2023)
-
Papillary Thyroid Carcinoma with Desmoid-Like Fibromatosis: Double Trouble?
Endocrine Pathology (2022)
-
Overview of the 2022 WHO Classification of Thyroid Neoplasms
Endocrine Pathology (2022)
-
CTNNB1 mutations in papillary thyroid carcinoma with prominent myofibroblastic stromal component
Modern Pathology (2021)
-
Papillary thyroid carcinoma with prominent myofibroblastic stromal component: clinicopathologic, immunohistochemical and next-generation sequencing study of seven cases
Modern Pathology (2020)