Abstract
More than 85% of patients with T1 colorectal cancer have no lymph node metastasis and can be cured by endoscopic resection. To avoid unnecessary surgery after complete endoscopic resection, accurate histologic methods for evaluating resected specimens are needed to discriminate those at high risk for lymph node metastasis. A retrospective multi-institutional, cross-sectional study of 806 T1 colorectal cancer patients was conducted. A budding/sprouting score was incorporated for predicting lymph node metastasis in addition to other parameters, including the depth of submucosal invasion, histologic grade, and lymphovascular invasion. Lymph node metastasis was detected in 97 patients. Independent predictors of lymph node metastasis by multivariate analysis were depth of submucosal invasion ≥1000 μm (odds ratio (95% confidence interval)=5.56 (2.14–19.10)) and high-grade budding/sprouting (3.14 (1.91–5.21)). Among lesions with a depth of submucosal invasion ≥1000 μm, lymph node metastasis was detected in 59 (29%) of 207 patients with high-grade budding/sprouting, and in 34 (9%) of 396 with low-grade budding/sprouting. Lymph node metastasis was detected in only 4 (2%) of 203 lesions with a depth of submucosal invasion <1000 μm. Of these four tumors, three invaded lymphatic and/or venous vessels. Thus, the risk for lymph node metastasis can be classified into three groups: high risk with a depth of submucosal invasion ≥1000 μm and high-grade budding/sprouting, intermediate-risk with a depth of submucosal invasion ≥1000 μm and low-grade budding/sprouting, and low-risk with a depth of submucosal invasion <1000 μm. These findings revealed that a depth of submucosal invasion ≥1000 μm and high-grade budding/sprouting are powerful predictive parameters for lymph node metastasis in T1 colorectal cancer. This three-tier risk classification system will facilitate the decision for additional major surgery for T1 colorectal cancer patients after successful endoscopic treatment.
Similar content being viewed by others
Main
Colorectal cancer confined to the mucosa and that invading the submucosa is classified as Tis and T1, respectively, according to the Union for International Cancer Control TNM classification1 and, in Japan, is considered early colorectal cancer regardless of lymph node metastasis.2 In the 1950s, early colorectal cancer was mainly treated surgically. For polypoid tumors of the rectum and distal colon, however, local excision was performed to avoid major surgery that could impair quality of life after surgery.3 With the development and advances of endoscopic therapeutic techniques, polypectomy and endoscopic mucosal resection have become important tools for treating early colorectal cancer without lymph node metastasis.4, 5, 6
When histopathologic examination of tumors removed by endoscopic procedures indicates invasion into the submucosa (T1 colorectal cancer), additional major surgery is recommended because of the risk of lymph node metastasis.7 The frequency of lymph node metastasis, however, is <15% based on large-scale studies,8, 9, 10, 11, 12, 13, 14, 15 and 85% of T1 colorectal cancer patients are cured by endoscopic treatment alone. The establishment of reliable criteria for discriminating patients with a high risk of lymph node metastasis from those with a low risk is thus an important issue in terms of providing adequate treatment to patients with T1 colorectal cancer.
To establish reliable criteria for recommendation of additional major surgery after endoscopic treatment in patients with T1 colorectal cancer, the Japanese Society for Cancer of the Colon and Rectum conducted a retrospective multicenter study to evaluate the risk of lymph node metastasis in T1 colorectal cancer patients.
Materials and methods
Patients
Patients with T1 colorectal cancer who underwent major surgery with or without preceding endoscopic resection between 1976 and 2007 at six institutions (National Defense Medical College, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, Dokkyo Medical University, Tokyo Medical and Dental University, Fukuoka University Chikushi Hospital, and Niigata University) affiliated with the Japanese Society for Cancer of the Colon and Rectum were enrolled in the study. Patient age, sex, tumor location, and lymph node metastasis status were obtained from the pathology records at each hospital. All procedures involving human participants in the present study were performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Histologic Evaluation
Histologic evaluations were performed by a single pathologist at each institute (HU, TN, TF, HK, AI, and YA) using hematoxylin and eosin-stained sections. Before beginning the evaluation process, the criteria for histologic evaluation were discussed in the Study Group for Budding/Sprouting in Colorectal Cancer of the Japanese Society for Cancer of the Colon and Rectum and a consensus was reached, as described below.
Budding/sprouting was assessed based on the number of foci of isolated cancer cells or a cluster comprising <5 cells in the invasive frontal region, as previously described by Ueno et al.13 The whole area of the invasive frontal region was scanned at low-power magnification, and then the field in which budding/sprouting was most intensive was selected. Budding/sprouting was counted in a field measuring 0.95 mm2 using a 20 × objective lens and 10 × ocular lens (Figure 1), and classified as grade 1 (0–4 foci in the field), grade 2 (5–9 foci), or grade 3 (≥10 foci; Figure 2). When the foci were difficult to be discriminated from mesenchymal cells such as fibroblasts or macrophages, or the foci were associated with marked inflammation or disrupted glands, such foci were excluded from being considered budding/sprouting foci to avoid overestimation.
Histologic assessment of budding/sprouting for T1 colorectal cancer is shown. Invasive frontal region is indicated by the dotted line. The whole area of the invasive frontal region was scanned at low-power magnification (a). One microscopic field containing the most intensive budding/sprouting foci was selected and the number of budding/sprouting foci was counted using 20 × objective lens and 10 × ocular lens (b).
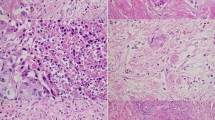
Representative histologic micrographs of budding/sprouting grade 2 (a, b) and grade 3 (c, d) are shown ((a, c) low-power magnification and (b, d) high-power magnification of the squares in (a) and (c) respectively). In (d), a representative budding/sprouting focus (indicated by arrow) is shown in the inset. Both grades 2 and 3 were also classified as ‘high-grade budding/sprouting’ in the present study.
The depth of submucosal invasion was measured according to the definition of the Japanese Society for Cancer of the Colon and Rectum (Figure 3).2, 16 First, each tumor was classified as one of the following three tumor types according to the tumor shape and status of the muscularis mucosa: pedunculated tumor, nonpedunculated tumor with identifiable muscularis mucosa, and nonpedunculated tumor without identifiable muscularis mucosa. The depth of submucosal invasion was measured according to the criteria for each tumor type. In pedunculated tumors, the depth of submucosal invasion was classified as head invasion (invasive cancer tissue was confined to the head of the polyp; corresponding to Haggitt’s level 1) or stalk invasion (cancer invaded into the stalk of the polyp; corresponding to Haggitt’s level 2 or deeper).17 In tumors with head invasion, the depth of submucosal invasion was considered to be 0 μm. In tumors with stalk invasion, the vertical distance from the line between the head and stalk (named ‘Haggitt’s line’ by Matsuda et al18) to the invasive front was measured as the depth of submucosal invasion. In nonpedunculated tumors with identifiable muscularis mucosa, the depth of submucosal invasion was defined from the bottom line of the muscularis mucosa to the invasive front. In nonpedunculated tumors without identifiable muscularis mucosa, the depth of submucosal invasion was defined as the tumor thickness measured from the surface of the tumor to the invasive front at the deepest invasive site. Histologic grade was classified into two levels, low grade (well differentiated to moderately differentiated) or high grade (poorly differentiated, mucinous carcinoma, or signet-ring cell carcinoma), and the most predominant component was considered the histologic grade of each tumor, using the criteria of the Japanese Society for Cancer of the Colon and Rectum.2 Lymphatic and venous invasion within the tumor as well as in the adjacent tissue was evaluated based on hematoxylin and eosin-stained sections and graded as positive or negative.
Statistical Analyses
The χ2 test or Wilcoxon test was used for analysis of the differences between pairs of groups. Univariate and multivariate logistic regression analyses were used to examine the risk factors for lymph node metastasis. In the multivariate analysis, all of the factors were placed in the model simultaneously. Two-sided P-values of <0.05 were considered statistically significant. JMP software (version 9.0.2; SAS Institute, Cary, NC, USA) was used for all statistical calculations.
Results
Subjects
A total of 806 tumors from 806 patients (482 men and 324 women, mean age 63.9 years, range 24–94 years) enrolled in the study were used.
Univariate and Multivariate Analyses of Lymph Node Metastasis
The results of the univariate analyses are summarized in Table 1. Lymph node metastasis was detected in 97 (12%) of 806 cases. The rate of lymph node metastasis in nonpedunculated-type tumors (91/667, 14%) was significantly greater than in pedunculated-type tumors (6/139, 4%). The depth of submucosal invasion was significantly greater in lesions with lymph node metastasis than in those without lymph node metastasis. Among 203 tumors with a depth of submucosal invasion <1000 μm, 4 (2%) had lymph node metastasis; this rate was significantly lower than the rate of lymph node metastasis in tumors with a depth of submucosal invasion ≥1000 μm (93/603, 15%; P<0.0001). The rate of lymph node metastasis of tumors with a high histologic grade was significantly greater than that of tumors with a low histologic grade (30% and 12%, respectively, P=0.01). Lymphatic and venous invasion were significantly correlated with lymph node metastasis. Tumors with budding/sprouting grade 2 or 3 were more frequently associated with lymph node metastasis than tumors with budding/sprouting grade 1 (25% and 7%, respectively, P<0.0001). The positive rate of lymph node metastasis did not differ significantly between budding/sprouting grade 2 and grade 3. According to the budding/sprouting findings, two-tier grading, low grade (corresponding to grade 1) and high grade (corresponding to grades 2 or 3), was used for subsequent analyses. The positive rate of lymph node metastasis was not dependent on age, sex, tumor location, or tumor size. Multivariate analysis by a logistic regression model using the six parameters that were significantly correlated with lymph node metastasis in the univariate analyses indicated that lymph node metastasis independently correlated with a depth of submucosal invasion ≥1000 μm and high-grade budding/sprouting (Table 2).
Combination of Risk Factors Based on the Results of Multivariate Analysis and the Frequency of Lymph Node Metastasis
Relationships between combinations of histologic parameters and lymph node metastasis were analyzed (Table 3). Among patients having tumors with both of the independent parameters (depth of submucosal invasion ≥1000 μm and high-grade budding/sprouting), 29% (59/207) had lymph node metastasis. On the other hand, no lymph node metastasis was detected in patients with tumors with a depth of submucosal invasion <1000 μm and high-grade budding/sprouting (0/26), and only a low rate of lymph node metastasis was observed in those with tumors with a depth of submucosal invasion <1000 μm and low-grade budding/sprouting (4/177, 2%). In these three groups, the rates of lymph node metastasis were not affected by histologic grade or lymphovascular invasion. In patients having tumors with a depth of submucosal invasion ≥1000 μm and low-grade budding/sprouting, 34 (9%) of 396 patients had lymph node metastasis. In this group, the rate of lymph node metastasis was higher in patients with either high histologic grade or positive lymphovascular invasion than in patients without either of these two parameters (20/160, 13%; vs 14/236, 6%; P=0.02).
Correlation between Tumor Type, Depth of Submucosal Invasion, Budding/Sprouting, and Lymph Node Metastasis
The rate of lymph node metastasis at each level of depth of submucosal invasion and each grade of budding/sprouting among pedunculated tumors and nonpedunculated tumors is shown in Table 4. In pedunculated tumors, the rates of lymph node metastasis in tumors with a depth of submucosal invasion ≥1000 μm and in tumors with high-grade budding/sprouting tended to be higher than in tumors with a depth of submucosal invasion <1000 μm and in tumors with low-grade budding/sprouting, respectively, although there was no statistical significance. In nonpedunculated tumors, tumors with a depth of submucosal invasion ≥1000 μm and tumors with high-grade budding/sprouting had significantly higher rates of lymph node metastasis than tumors with a depth of submucosal invasion <1000 μm and tumors with low-grade budding/sprouting, respectively (P<0.0001).
Discussion
The findings of the present study demonstrated that six parameters, including nonpedunculated tumor type, depth of submucosal invasion ≥1000 μm, high histologic grade, positive lymphatic invasion, positive venous invasion, and high-grade budding/sprouting, were significant predictive factors for lymph node metastasis by univariate analysis, and depth of submucosal invasion ≥1000 μm and high-grade budding/sprouting were statistically independent factors by multivariate analysis. Based on these findings, we stratified the risk of lymph node metastasis into three groups by combining independent factors: high-risk group: tumors with a depth of submucosal invasion ≥1000 μm, and high-grade budding/sprouting (lymph node metastasis present in 29%); intermediate-risk group: tumors with a depth of submucosal invasion ≥1000 μm and low-grade budding/sprouting (lymph node metastasis in 9%); and low-risk group: depth of submucosal invasion <1000 μm (lymph node metastasis in 2%). Histologic grade and lymphovascular invasion might be useful determinants for classifying the intermediate-risk group. Although histologic grade and lymphovascular invasion were not significant factors in the high-risk and low-risk groups, three of four cases with lymph node metastasis in the low-risk group had lymphatic and/or venous invasion.
Histologic risk factors for lymph node metastasis in T1 colorectal cancer have been reported by many studies and are used for decisions regarding treatment for patients undergoing endoscopic excision of T1 colorectal cancer. The National Comprehensive Cancer Network guidelines and the European Society for Medical Oncology guidelines recommend that patients with tumors having at least one of the histologic risk factors (unfavorable histologic features) should undergo additional major surgery.19, 20, 21 In Japan, the guidelines first published in 2005 by Japanese Society for Cancer of the Colon and Rectum recommended additional major surgery after successful endoscopic excision when tumors had at least one of the risk factors, that is, a depth of submucosal invasion ≥1000 μm, high histologic grade (poorly differentiated, mucinous carcinoma, or signet-ring cell carcinoma), or lymphovascular invasion.22 In the second edition (Japanese Society for Cancer of the Colon and Rectum guidelines 2010), budding/sprouting was incorporated into the histologic risk factors.16 In patients receiving additional major surgery according to the recommendation, however, metastasis was found in 15 to 16%, and up to 80% or more might undergo unnecessary surgery in terms of lymph node metastasis.15, 23
According to the recommendation of the Japanese Society for Cancer of the Colon and Rectum guidelines 2010, additional major surgery would not be recommended for 121 (15%) of the 806 patients in the present study and lymph node metastasis could be found in only 1 (1%), whereas additional surgery would be recommended for 685 patients (85%) and lymph node metastasis could be found in 96 (14%). This concept may be beneficial but still many patients would have unnecessary surgery. Regarding international consensus guidelines such as those of the National Comprehensive Cancer Network or European Society for Medical Oncology,19, 20, 21 similar results might be obtained based on a similar concept. To further reduce unnecessary surgery, more useful methods for predicting the risk of lymph node metastasis in patients with T1 colorectal cancer are needed. Based on stratification of the risk group of the present study, additional surgery would not be recommended for 203 (25%) patients in the low-risk group, and endoscopic treatment alone would be curative in 236 (29%) patients in the intermediate-risk group with neither high histologic grade nor lymphovascular invasion.
Several studies of the risk of lymph node metastasis in T1 colorectal cancer revealed a predictive value of each histologic parameter, but only a few studies conducted to date have established effective risk evaluation methods for lymph node metastasis in T1 colorectal cancer. Ueno et al13 proposed predictive models based on the analysis of 251 cases, and demonstrated that a combination of unfavorable histologic grade, vascular invasion, budding/sprouting, and width of submucosal invasion was the best model. Egashira et al24 analyzed 140 cases and developed an effective algorithm based on parameters such as the presence and depth of lymphatic permeation, depth of submucosal invasion, structural atypia (cribriform type), lymphoid infiltration, and venous invasion. Compared with these studies, the present study was based on a larger number of cases and used standardized methods to evaluate the depth of submucosal invasion and budding/sprouting to develop a routine pathologic examination for stratifying patients based on the risk of lymph node metastasis. Although further investigation is necessary to validate these algorithms, the present study provides useful information for determining whether additional surgery is recommended for patients with T1 colorectal cancer removed by an endoscopic approach.
The present study demonstrated that budding/sprouting is a high impact factor for predicting lymph node metastasis in T1 colorectal cancer. Although the implication of budding/sprouting in deeply invasive colorectal cancer was first reported in 1989,25 many studies have also focused on budding/sprouting as a risk factor of lymph node metastasis in T1 colorectal cancer.12, 13, 14, 15, 26, 27, 28, 29, 30, 31, 32 Some investigators refer to findings similar to budding/sprouting as ‘unfavorable histology at the invasive front,’33 ‘focal dedifferentiation,’34 or ‘tumor cell dissociation,’35 although the definition is not always consistent.13, 25, 32, 36 In the evaluation of budding/sprouting, we adopted the definition of Ueno et al13 because it is widely used and has good reproducibility.13, 37 The present study originally classified budding/sprouting into grades 1, 2, and 3 based on the number of budding/sprouting foci. The two-tier grading system using low grade (corresponding to grade 1) and high grade (corresponding to grades 2 or 3) however is better for practical use because there were no significant differences between grades 2 and 3 in terms of lymph node metastasis in the present study.
Regarding tumor type, pedunculated tumors are considered to have a relatively low frequency of lymph node metastasis. Matsuda et al18 reported an overall incidence of lymph node metastasis in 3.5% in such tumors, similar to our results (4%). Moreover, tumors with head invasion and stalk invasion with a depth of invasion <1000 μm are considered to have an extremely low frequency of lymph node metastasis,12, 18 as confirmed by the present study. We also elucidated that the cutoff value of 1000 μm for nonpedunculated tumors was very useful, as previously reported.12, 14 That is, this cutoff value seems feasible for practical use in both pedunculated and nonpedunculated tumors. The present study revealed that tumors with a depth of submucosal invasion ≥1000 μm and high-grade budding/sprouting had a higher incidence of lymph node metastasis among both pedunculated and nonpedunculated tumors. In pedunculated tumors, however, the difference was not statistically significant and this may be because of the low overall incidence of lymph node metastasis.
In conclusion, the present study revealed that depth of submucosal invasion ≥1000 μm and budding/sprouting were powerful predictive parameters for lymph node metastasis in T1 colorectal cancer. The three-tier risk classification proposed in the present study might provide important information for deciding on treatment for T1 colorectal cancer patients after endoscopic excision.
References
Sobin LH, Gospodarowicz MK, Wittekind C (eds). The TNM Classification of Malignant Tumors, 7th edn. Wiley-Blackwell: Oxford, 2009, pp 100–105.
Japanese Society for Cancer of the Colon and Rectum (ed). Japanese Classification of Colorectal Carcinoma, 2nd English Edition. Kanehara: Tokyo, 2009 pp 6–7, 37-39, 57.
Lockhart-Mummery HE, Dukes CE . The surgical treatment of malignant rectal polyps. Lancet 1952;260:751–755.
Williams C, Muto T, Rutter KR . Removal of polyps with fibreoptic colonoscope: a new approach to colonic polypectomy. Br Med J 1973;1:451–452.
Kudo S . Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993;25:455–461.
Karita M, Tada M, Okita K et al. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc 1991;37:128–132.
Colacchio TA, Forde KA, Scantlebury VP . Endoscopic polypectomy: inadequate treatment for invasive colorectal carcinoma. Ann Surg 1981;194:704–707.
Nascimbeni R, Burgart LJ, Nivatvongs S et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002;45:200–206.
Sakuragi M, Togashi K, Konishi F et al. Predictive factors for lymph node metastasis in T1 stage colorectal carcinomas. Dis Colon Rectum 2003;46:1626–1632.
Yamamoto S, Watanabe M, Hasegawa H et al. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology 2004;51:998–1000.
Okabe S, Shia J, Nash G et al. Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg 2004;8:1032–1039.
Kitajima K, Fujimori T, Fujii S et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534–543.
Ueno H, Mochizuki H, Hashiguchi Y et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004;127:385–394.
Tateishi Y, Nakanishi Y, Taniguchi H et al. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol 2010;23:1068–1072.
Nakadoi K, Tanaka S, Kanao H et al. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol 2012;27:1057–1062.
Watanabe T, Itabashi M, Shimada Y et al. Japanese Society for Cancer of Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012;17:1–29.
Haggitt RC, Glotzbach RE, Soffer EE et al. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985;89:328–336.
Matsuda T, Fukuzawa M, Uraoka T et al. Risk of lymph node metastasis in patients with pedunculated type early invasive colorectal cancer: a retrospective multicenter study. Cancer Sci 2011;102:1693–1697.
NCCN clinical practice guidelines in oncology; colon cancer version 3.2014 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 12 February 2014.
NCCN clinical practice guidelines in oncology; rectal cancer version 3.2014 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 12 February 2014.
Labianca R, Nordlinger B, Beretta GD et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi64–vi72.
Japanese Society for Cancer of the Colon and Rectum (ed). Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2005 for the Treatment of Colorectal Cancer. Kanehara: Tokyo, 2005, in Japanese.
Morson BC . Factors influencing the prognosis of early cancer of the rectum. Proc R Soc Med 1966;59:607–608.
Egashira Y, Yoshida T, Hirata I et al. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol 2004;17:503–511.
Morodomi T, Isomoto H, Shirouzu K et al. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer 1989;63:539–543.
Araki Y, Isomoto H, Shirouzu K et al. Clinicopathological characteristics of colorectal submucosal carcinoma with lymph node metastasis. Kurume Med J 1993;40:123–127.
Ogawa T, Yoshida T, Tsuruta T et al. Tumor budding is predictive of lymphatic involvement and lymph node metastasis in submucosal invasive colorectal adenocarcinomas and in non-polypoid compared polypoid growths. Scand J Gastroenterol 2009;44:605–614.
Choi DH, Sohn DK, Chang HJ et al. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum 2009;52:438–445.
Yasuda K, Inomata M, Shiromizu A et al. Risk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resection. Dis Colon Rectum 2007;50:1370–1376.
Beaton C, Twine CP, Williams GL et al. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 2013;15:788–797.
Bosch SL, Teerenstra S, de Wilt JH et al. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013;45:827–834.
Shimomura T, Ishiguro S, Konishi H et al. New indication for endoscopic treatment of colorectal carcinoma with submucosal invasion. J Gastroenterol Hepatol 2004;19:48–55.
Masaki T, Muto T . Predictive value of histology at the invasive margin in the prognosis of early invasive colorectal carcinoma. J Gastroenterol 2000;35:195–200.
Tominaga K, Nakanishi Y, Nimura S et al. Predictive histopathologic factors for lymph node metastasis in patients with nonpedunculated submucosal invasive colorectal carcinoma. Dis Colon Rectum 2005;48:92–100.
Hori H, Fujimori T, Fujii S et al. Evaluation of tumor cell dissociation as a predictive marker of lymph node metastasis in submucosal invasive colorectal carcinoma. Dis Colon Rectum 2005;48:938–945.
Hase K, Shatney C, Johnson D et al. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 1993;36:627–635.
Mitrovic B, Schaeffer DF, Riddel RH et al. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol 2012;25:1315–1325.
Acknowledgements
We thank Dr Tetsuji Yokoyama for assistance of the statistical analysis. This study was financially supported by the Japanese Society for Cancer of the Colon and Rectum.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kawachi, H., Eishi, Y., Ueno, H. et al. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol 28, 872–879 (2015). https://doi.org/10.1038/modpathol.2015.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2015.36
This article is cited by
-
ARL4C is associated with epithelial-to-mesenchymal transition in colorectal cancer
BMC Cancer (2023)
-
A proposal for grading the risk of lymph node metastasis after endoscopic resection of T1 colorectal cancer
International Journal of Colorectal Disease (2023)
-
Prediction of lymph node metastasis in early colorectal cancer based on histologic images by artificial intelligence
Scientific Reports (2022)
-
The essential problem of over-measuring the depth of submucosal invasion in pT1 colorectal cancer
Virchows Archiv (2022)
-
Role of barium enema examination for the diagnosis of submucosal invasion depth in T1 colorectal cancers
Cancer Imaging (2021)