Abstract
Melanomas arising in association with blue nevi or mimicking cellular blue nevi comprise a relatively rare and heterogeneous group of melanomas. It remains controversial which prognostic indicators predictive of outcome in conventional cutaneous melanomas are applicable to this type of melanoma. Here, we describe the clinical and histopathologic features of 24 melanomas arising in association with blue nevi and correlate these with clinical outcome. The mean patient age was 49 years (range: 23–85) with a slight female predominance (15 females:9 males). The most common anatomic locations included the head and neck region (50%), the trunk (21%), and the buttock/sacrococcygeum (17%). Histologically, the tumors were typically situated in the mid to deep dermis with variable involvement of the subcutis, but uniformly lacked a prominent intraepithelial component. The mean tumor thickness (defined as either the standard Breslow thickness or, if not available due to the lack of orientation or lack of epidermis, the largest tumor dimension) was 20.9 mm (range: 0.6–130 mm). The mean mitotic figure count was 6.5/mm2 (range: 1–30/mm2). Perineural invasion was common (38%). Follow-up was available for 21 cases (median 2.1 years). The median overall survival, recurrence-free survival, time to local recurrence, and time to distant recurrence were 5.2, 0.7, 2.6, and 1.6 years, respectively. Logistic regression analyses demonstrated a significant association between tumor thickness and recurrence-free survival (hazard ratio=1.02 per mm; P=0.04) and reduced time to distant metastasis (hazard ratio=1.03 per mm; P=0.02) with a similar trend toward reduced time to local recurrence (hazard ratio=1.02 per mm; P=0.07). No other parameters (age, anatomic location, mitotic figures, lymphovascular or perineural invasion, or type of associated blue nevus) emerged as significant. In addition, we provide a comprehensive review of 109 cases of melanoma blue nevus type described in the English literature and summarize our findings in this context.
Similar content being viewed by others
Main
Blue nevi represent a broad spectrum of melanocytic proliferations with distinctive clinical and histopathologic features. Common blue nevi were first described by Jadassohn-Tieche.1 These nevi typically develop congenitally or de novo on the extremities, scalp, and buttocks and exhibit a characteristic blue–black-grey–black clinical discoloration of variable sizes.2, 3 Histopathologically, common blue nevi are characterized by a wedge-shaped, variably cellular dermal proliferation of distinctive spindled, dendritic melanocytes with elongated, hyperchromatic nuclei and variable amounts of coarse intracytoplasmic pigment. An associated desmoplastic stromal reaction and melanophages are typical.2, 3 Cellular blue nevi most commonly arise on the buttock/sacrococcygeal area and less commonly, the scalp and extremities. They typically grow as larger, multilobulated masses (often with a characteristic ‘dumbbell shape’) and consist of a biphenotypic proliferation of dendritic melanocytes reminiscent of classic blue nevus admixed with cellular nodules of spindled oval-shaped melanocytes with a variable admixture of pigmented macrophages.2, 3, 4 The term melanoma blue nevus type designates a heterogeneous group of malignant melanocytic proliferations (melanomas) that either (A) arise in association with common or cellular blue nevi or (B) develop de novo but architecturally or cytologically mimic cellular blue nevus.3, 4, 5 Diagnostic histopathologic features include a proliferation of melanocytes with malignant cytology (nuclear pleomorphism, coarse chromatin, prominent nucleoli), increased and/or atypical mitotic figures, tumor necrosis, and invasive and/or destructive pattern of growth and occasionally, an adjacent/associated proliferation of distinctly benign blue nevus dendritic melanocytes.6
Although melanoma blue nevus type have been shown to exhibit outcomes similar to conventional melanoma when matched for Breslow thickness, age, Clark level, and ulceration,7 it remains controversial which—if any—of the discrete clinical and pathologic indicators (like age, gender, Breslow thickness, mitotic figures, and ulceration) predictive of clinical outcome in conventional cutaneous melanomas might apply to this group of melanomas. This is especially contentious considering the typical restriction of melanoma blue nevus type to the dermis and/or subcutaneous tissue without an associated intraepidermal component, which has been suggested to undermine the biological relevance of Breslow thickness and tumor ulceration as predictors of outcome.5 Further complicating this issue, the constellation of distinctive diagnostic features described above is not apparent in every case; thus, the unequivocal distinction of melanoma (arising with or resembling blue nevus) from benign cellular blue nevus or atypical cellular blue nevus8, 9 also remains controversial—even among experienced dermatopathologists.10 It is therefore not surprising that there are only ∼100 cases described in the literature to date4, 5, 7, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 and the majority of these are individual case reports or small case series—too few to achieve statistical significance in correlating histopathologic features with clinical behavior.
Here, we describe the clinical and histopathologic features of 24 melanomas arising in association with common or cellular blue nevi. We further correlate the clinical and histopathologic features of these lesions with annotated clinical follow-up information. We demonstrate a statistically significant association between tumor thickness (defined as either the standard Breslow thickness or, if not available due to lack of orientation or lack of epidermis, the largest tumor dimension) and both recurrence-free survival and reduced time to distant metastasis. In addition, we provide a comprehensive review of the 109 cases of melanoma blue nevus type described in the English literature and summarize our findings in the context of the clinical and pathologic features of these lesions.
Materials and methods
Patient Specimens
With approval from the Institutional review Board at the University of Texas, MD Anderson Cancer Center, the files of the Department of Pathology at University of Texas, MD Anderson Cancer Center were searched for cases of melanoma described as arising in association with a common or cellular blue nevus. Twenty-four cases of primary lesions in patients without prior history of invasive melanoma were identified from the past 9 years. In each case, either hematoxylin and eosin-stained slides or whole slide scanned images were reviewed to confirm the diagnosis and record the histopathologic features including tumor thickness, tumor cytology, number of mitotic figures, type of associated benign nevus, and the presence of an intraepidermal component, ulceration, regression, lymphovascular invasion, perineural invasion, satellitosis, and tumor necrosis. The criteria to distinguish between melanoma and an associated nevus component included distinctly biphenotypic foci with increased cellularity, prominent nuclear pleomorphism and nucleoli, increased mitotic figures, and the presence of coagulative tumor necrosis.6 ‘Tumor thickness’ was defined as the standard Breslow thickness when the lesion abutted or came in close proximity to the epidermis or the largest tumor dimension available for a given lesion (when the lesion grew as a nodule at a location distant from the epidermis and/or there was no epidermis available for evaluation in the specimen). Only the malignant component was measured when assessing maximum tumor thickness. Using this definition, clinical attributes and follow-up information for each case were obtained from electronic medical records, when available.
Mutation Analysis
BRAF mutation analysis was performed using targeted next generation sequencing or pyrosequencing as previously described.55, 56 GNAQ mutation analysis was performed using DNA extracted from unstained formalin fixed, paraffin-embedded tissue sections. We tested for mutations in exon 5, codon 209 of the GNAQ gene using Sanger sequencing. Polymerase chain reaction amplification analyses of six samples were performed in duplicate. Polymerase chain reaction was performed in a 96-well plate with a 50 μl volume including 100 ng DNA, 10 μM of M13 tagged GNAQ Exon 5 forward and reverse primers, 25 μM MgCl2, 5 μl 10 × GoTaq buffer, 5 μl 10 mM dNTP mix, and 0.3 μl of 5 units/μl of GoTaq enzyme. The reaction mix was subjected to initial denaturation at 95°C for 10 minutes, followed by 40 cycles of amplification consisting of denaturation at 95 °C for 30 seconds, annealing at 55 °C for 30 seconds, and extension at 72 °C for 30 seconds followed by final extension at 72 °C for 7 min and hold at 4 °C. To facilitate sequencing of amplicons, the forward and reverse primers were tagged with M13 universal sequences: M13 forward, 5′-TGTAAAACGACGGCCAGT-3′ or M13 reverse, 5′-CAGGAAACAGCTATGACC-3′. PCR products were purified using AMPure magnetic beads (Agentcourt, Danvers, MA) according to the manufacturer’s protocol. A quantity of 4 μl of purified amplicons was used for Sanger sequencing using 3130 DNA Analyzer (Applied Biosystems, Carlsbad, CA) for detection of mutation in codon 209 of GNAQ Exon 5. The resulting data were analyzed by SeqScape software versions 2.5 and/or 2.7 (Applied Biosystems).
Statistical analysis
Four outcomes were interrogated: overall survival, recurrence-free survival, time to local recurrence, and time to distant recurrence. Cox proportional hazards regression models were fit to model the association between each survival parameter and clinical and tumor covariates of interest. No adjustment was made for the multiplicity of testing.
Results
Clinical Features
Clinical features of the patients are summarized in Table 1. Overall, there were 9 male and 15 female patients with a mean age of 49 years (range: 20–85 years; median: 50 years). The majority of patients were Caucasian (22/24; 92%). One patient was Black (4%) and one was Hispanic (4%). The tumors were located on the head and neck region in 12 patients (50%), the trunk in 5 patients (21%), and the buttock/sacrococcygeal region in 4 patients (17%); the perianal area (1 patient; 4%), the penis (1 patient; 4%), and the spinal cord (1 patient; 4%) were less common sites of involvement. No case arose on the extremities in our series.
Clinically, most patients (10/24; 42%) endorsed a history of a recently enlarging and/or changing pigmented lesion, which had been present for many years prior to the diagnosis. In patient 1, the lesion was identified during a routine dermatologic exam. Patient 2 presented with metastatic tumor. Patient 4 presented with blurry vision, and patient 14 presented with exophthalmos. Patient 16 was receiving chemotherapy (intraperitoneal and intravenous cisplatin and paclitaxel) for ovarian cancer and noticed a darkly pigmented lesion on her scalp after chemotherapy-induced alopecia. Patient 21 presented with sciatic nerve pain. Initial presenting clinical features were not available for the remaining eight patients.
Histopathologic Findings
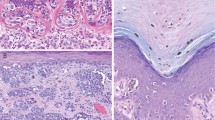
Histopathologic findings are summarized in Table 2. An example of melanoma blue nevus type situated in close proximity to the epidermis is shown in Figure 1. The lesions lacked a prominent intraepidermal component in all cases, except one (case 1). In addition, many cases arose in dermal and subcutaneous sites and were situated a significant distance from the epidermis. Figure 2 illustrates an example of a case arising in the perianal soft tissue where the largest tumor dimension was measured to represent tumor thickness due to lack of epidermis. Furthermore, in eight cases, the epidermis was not available for review; therefore, a calculation of Breslow thickness was not always feasible. The mean tumor thickness in our series was 20.9 mm (range: 0.6–130 mm; median 12 mm). Mitotic figures were identified in all cases; the mean mitotic figure count was 6.5/mm2 (range: 1–30/mm2; median 3). Perineural invasion was present in nine cases (38%) and satellitosis was identified in four cases (17%). In contrast, despite the overall relatively large size of the lesions, lymphovascular invasion (three cases; 13%), regression (two cases; 8%), and ulceration (one case; 4%) were comparatively rare phenomena in this series of melanomas.
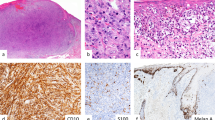
Melanoma arising in association with common and cellular blue nevus on the scalp of a 49-year-old woman (case 16). (a) Shave biopsy specimen of a pigmented lesion reported to have been present for years on the scalp of a 49-year-old female reveals epidermal ulceration and a proliferation of cytologically benign spindled and dendritic melanocytes throughout the subjacent dermis (H&E, × 40). (b) Higher power examination of the lesion reveals attributes of a proliferation of benign spindled melanocytes (H&E, × 400). (c) Excision of the lesion site 2 months later reveals an asymmetric, distinctly biphenotypic proliferation of markedly atypical melanocytes forming an expansile and infiltrative nodule in the dermis with extension into the subcutaneous adipose tissue to a depth of ∼10.8 mm arising in association with a benign blue nevus component (H&E × 20). (d) Common and cellular blue nevus components are associated with the melanoma and exhibit a benign spindled morphology similar to that seen in the original shave biopsy specimen with a plate-like and dumbbell-shaped architecture (H&E, × 100; inset; H&E × 200). (e) Cytologically malignant melanocytes proliferate adjacent to the blue nevus component and form an expansile nodule with central necrosis. These cells exhibit an epithelioid morphology with increased pale amphophilic cytoplasm and enlarged oval nuclei with prominent nucleoli. Mitotic figures are present (H&E, × 100; inset: × 400).
Melanoma arising in association with a common blue nevus in the perianal soft tissue of a 67–year-old man (case 24). (a) Excisional biopsy specimen of a pigmented lesion arising in the perianal soft tissue of a 67–year-old male reveals a distinctly biphenotypic melanocytic proliferation comprising a centrally located nodular proliferation of atypical melanocytes with a surrounding less cellular spindled melanocytic proliferation. (H&E, × 20). (b) Higher power examination of the lesion at the border of the biphenotypic proliferation reveals benign spindled melanocytes abutting a more cellular proliferation of atypical epithelioid melanocytes (H&E, × 100). (c) Higher power examination of the spindled area reveals a proliferation of benign spindled melanocytes associated with dense fibrosis and pigmented macrophages (H&E, × 200). (d) Cytologically malignant melanocytes forming an expansile nodule. These cells exhibit an epithelioid morphology with increased pale amphophilic cytoplasm and enlarged oval nuclei with prominent nucleoli. Mitotic figures are present (H&E, × 100; inset: × 400).
In the cases for which we were able to assess cytomorphologic features, the tumor cells ranged from purely malignant epithelioid melanocytes in eight cases (33%) to a combination of malignant epithelioid and spindled melanocytes in 12 cases (50%). Twenty-three cases contained an unequivocal benign nevus component: six cases with common blue nevus (25%), seven cases with cellular blue nevus (29%), nine cases with both common and cellular blue nevus (38%), and one case with blue nevus/neurocristic hamartoma (4%). In one case (4%), the melanoma was present in association with an atypical cellular blue nevus. The benign component ranged from focal (≤20% of the lesion) and present only at the periphery of the lesion (14 cases), to comprising ∼20–50% of the lesion (seven cases), to comprising ≥50% of the proliferation with only a focal malignant component (in three cases; most prominent in case 21). The transition between the benign and malignant components was typically abrupt. The presence of the benign blue nevus areas in our case series was critical in the distinction of primary tumors from metastases.
Molecular Data
Tumors from four patients were analyzed for mutations in the BRAF gene; all were wild type for BRAF. An additional five samples were tested for GNAQ mutation; all cases were wild type for GNAQ. One sample was tested for both BRAF and GNAQ mutations and was wild type for both genes.
Clinical Follow-up and Outcome
Clinical follow-up was available for 21 patients. Overall, follow-up times ranged from 0.04 to 11.6 years with a median of 2.1 years.
Fifteen patients (15/24: 63%) underwent sentinel lymph node biopsy. Among these, four (4/15: 27%) had negative sentinel nodes and did not develop subsequent regional or distant metastasis (mean 1.62 years; range: 0.04–5.2 years). Six patients (6/15: 40%) initially had negative sentinel lymph nodes, but later developed either regional metastasis (one patient (1/15: 7%) with microscopic satellitosis concurrent with negative sentinel lymph node biopsy) or subsequent distant metastases, five patients (5/15: 33%): involving the lung, liver, mediastinal lymph nodes, and subcutaneous soft tissue in order of frequency; mean 3.2 years; range: 0.1–10.0 years). Finally, sentinel lymph node involvement was detected for five patients (5/15: 33%); among these, two (2/15:13%) subsequently developed additional regional metastases (involving the regional lymph nodes; 0.1 years later each), and three patients (3/15: 20%) developed both regional lymph node and distant metastases (involving the liver, renal parenchyma, and subcutaneous soft tissue; mean 0.3 years to first event; range: 0–1.0 years after diagnosis).
An additional six patients (6/24: 25%) did not undergo sentinel lymph node sampling but had follow-up information available. Among these, two patients (2/6: 33%) developed neither local recurrence nor regional or distant metastasis; three patients (3/6: 50%) developed only distant metastases (mean 2.9 years; range: 0–8.5 years); and one patient (1/6: 17%) developed both regional and distant metastases (0.7 years from diagnosis).
Distant metastatic sites most commonly included the liver (8/12: 67%); the lung (4/12: 33%); subcutaneous tissue (3/12: 25%); bone (2/12: 17%); kidney (2/12: 17%); distant lymph nodes (2/12: 17%); abdomen (1/12: 8%); spleen (1/12: 8%); adrenal glands (1/12: 8%) and retroperitoneum (1/12: 8%). Seven patients (29%) had died at the time of our study; among these 6 (25%) died of disease with documented distant metastasis. The cause of death for one patient was unknown.
Statistical Analysis and Risk Stratification
Detailed results from univariate Cox proportional hazards regression models for time to distant metastasis and recurrence-free survival are presented in Tables 3 and 4, respectively. These demonstrate a significant association between tumor thickness and recurrence-free survival (hazard ratio=1.02 per mm; P=0.04) and reduced time to distant metastasis (hazard ratio=1.03 per mm; P=0.02) with a similar trend toward reduced time to local recurrence (hazard ratio=1.02 per mm; P=0.07). No other parameter (including age, anatomic location, mitotic figures, lymphovascular or perineural invasion, necrosis, or type of associated blue nevus) emerged as significant. None of the covariates of interest were significantly associated with overall survival (data not shown).
Discussion
In the current study, we describe the clinical and histopathologic features of 24 melanomas arising in association with common or cellular blue nevi and correlate these features with annotated clinical follow-up information. Ours is the first study to demonstrate a significant association between the variable of tumor thickness (defined as either the standard Breslow thickness or, if not available due to lack of orientation or lack of epidermis, the largest dimension) with both recurrence-free survival and reduced time to distant metastasis; other prognostic indicators predictive of outcome in conventional cutaneous melanomas did not correlate with outcome in these rare and unique melanomas.
Since the first description of melanomas arising in association with or mimicking blue nevi, there has been controversy regarding whether clinical and/or histopathologic prognostic indicators (age, gender, Breslow thickness, mitotic figures, and ulceration) that are predictive of clinical outcome in conventional cutaneous melanomas might also apply to this group of melanomas. For example, because the vast majority of standard cutaneous melanomas arise in association with an intraepidermal component, the Breslow thickness (tumor depth from the anatomic boundary of the epidermis) is accepted (and proven) to accurately reflect a given lesion’s intrinsic propensity for clinically aggressive behavior.57 In contrast, the tendency of melanoma blue nevus type to arise as nodules—either in the dermis or in the subcutaneous tissue without an associated intraepidermal component and thus, without a true epidermal origin—calls into question whether a measurement of Breslow thickness accurately captures such a lesion’s true propensity for biologically aggressive/invasive behavior. Further complicating this issue is the relative paucity of cases described in the literature and the lack of consensus regarding the diagnostic criteria.5 In a study by Martin et al,7 the authors described the clinical and histopathologic characteristics of 23 ‘blue nevus-like melanomas’ and demonstrated that these lesions do not exhibit differences in survival or an increased risk of lymph node or distant metastasis when compared with conventional melanomas matched for age, sex, Breslow thickness, Clark level, ulceration, and anatomic site. Moreover, they demonstrated a statistically significant difference (P=0.002) between the mean Breslow thickness of lesions in patients who developed locally recurrent disease (mean Breslow thickness=9.4 mm) and those who did not (mean Breslow thickness=4.2 mm), while other histopathologic features were not predictive of outcome in these lesions.7
Our findings underscore and expand upon these findings. Namely, we show that increasing tumor thickness (which we defined as either the Breslow thickness, when applicable, or the largest tumor dimension available for lesions growing as more deeply situated nodules) correlates with recurrence-free survival (hazard ratio=1.02 per mm; P=0.04) and reduced time to distant metastasis (hazard ratio=1.03 per mm; P=0.02) with a similar trend toward reduced time to local recurrence (hazard ratio=1.02 per mm; P=0.07). Taken together with the results of Martin et al,7 our findings argue that the largest tumor dimension is an important variable to capture in the synoptic report—particularly for deeply situated lesions of this sort. We did not observe a statistically significant relationship between other parameters (including age, gender, mitotic figures, tumor necrosis, and lymphovascular invasion) and clinical outcome in our series. An additional interesting observation in our study was the finding that despite the relatively high mitotic figure count (mean 6.5/mm2 for all cases) and the relatively large size of these lesions (mean tumor thickness: 20.9 mm), ulceration was a relatively rare event compared with conventional nodular melanomas with similar size and mitotic figure count.58, 59, 60, 61 This observation may be attributable to the fact that many of our lesions arose in the deep dermis/subcutis, supporting the concept that ulceration is likely related to the relationship/proximity of the lesion to the epidermis. Alternatively, recent gene expression profiling studies have shown that ulcerated primary melanomas have distinct gene expression profiles compared with their non-ulcerated counterparts, suggesting that ulceration may in fact be driven by properties intrinsic to the tumor.62
To further our understanding of melanomas arising in association with or mimicking blue nevi, we performed a comprehensive review of the literature to systematically classify the clinical–pathologic characteristics of these lesions and to contextualize the cases we describe. We identified 109 melanoma blue nevus type described between 1953 and 2012 in the English literature either as individual case reports or in case series, further underscoring the relatively rare nature of this entity (Figure 3).4, 5, 7, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 Overall, patient age ranged from 3 to 77 years5, 48 with a median age of 44.7 years similar to what we report here (range: 20–85 years; median 50 years; Figure 3b). Overall, there is a slight male preponderance described in the literature (56 lesions in males compared with 51 women)—in contrast to our findings (9 men to 15 women). The most common anatomic site for these lesions is the head and neck region in 52 cases,4, 5, 7, 11, 13, 15, 16, 17, 18, 19, 20, 21, 23, 24, 27, 28, 31, 33, 36, 39, 42, 46, 47, 48, 51, 53 followed by the trunk in 28 cases,4, 5, 7, 11, 12, 13, 16, 18, 25, 32, 34, 35, 38, 43, 45, 50, 52 the lower extremity in 11 cases,4, 7, 13, 14, 26, 30, 33 the upper extremity in 8 cases,7, 13, 18, 29, 40 the buttock region in 7 cases,4, 5, 16, 33, 37 the perineal region in 2 cases,22, 49 and the uterine cervix in 1 case.41 We observed a similar anatomic distribution in our cohort of cases (Table 1); however, none of our cases occurred on the extremities—a finding identical to what Granter et al5 reported in their series. This significant predilection for the head and neck supports our standard recommendation that incompletely excised/sampled melanocytic lesions with any features of blue nevus arising in this anatomic site that extensively involve the tissue edges warrant at least conservative re-excision to ensure complete removal and to completely evaluate these lesions—particularly those lesions with a reported history of changing appearance (Figure 1).
Associated nevus component (a), age (b), gender (c), and anatomic distribution (d) for all reported cases in the English literature, to date, combined with our data from this study. Not every parameter is provided for each reported case in the literature and therefore, the figures represent cases for which the specific respective parameters were reported. (a) Provides a breakdown of the associated nevus component for our cases as well as those reported in the literature (n=108)4, 5, 7, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 (b) Age (n=111),4, 5, 7, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 (c) gender (n=131)4, 5, 7, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 and (d) anatomic distribution (n=135)4, 5, 7, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 for all cases.
In contrast to our findings, in their series of 23 patients Martin et al7 showed that blue nevus-like melanoma was more common in men and demonstrated an even distribution among body sites (ie no obvious predilection for the head and neck). The median age of patients at diagnosis in their study was similar to what we observed (∼5th decade). The reported median tumor thickness in their series (5.5 mm) was less than what we found in our cases (12.0 mm) with the caveat that (1) they used Breslow thickness and we used the greatest tumor dimension when Breslow thickness was not applicable and (2) our cases are derived from the experience at a tertiary cancer referral center that might produce a selection bias for larger lesions that are more difficult to treat and/or exhibit clinically aggressive behavior.
Regarding pathologic findings, the reported cases (in order of frequency) included malignant melanocytic proliferations mimicking a cellular blue nevus but lacking an identifiable benign component (in 38% of cases)5, 11, 13, 14, 15, 18, 20, 25, 28, 30, 33, 36, 47, 49 or melanomas arising in association with a distinctive blue nevus component, including (a) cellular blue nevus (26%),4, 5, 11, 16, 18, 29, 40, 46, 50, 51, 53 (b) common blue nevus (21%),5, 13, 22, 24, 27, 31, 32, 34, 37, 39, 41, 43, 52 (c) both cellular and common blue nevus (13%),12, 16, 21, 23, 35, 38, 44, 45, 48 (d) neurocristic hamartoma (1%)42 and (e) nevus of Ota (1%).17 In contrast, all of the cases in our series consisted of distinctly biphenotypic melanocytic proliferations (melanoma together with a benign blue nevus component). The most common associated blue nevus pattern in our series was both common and cellular blue nevus (nine cases; 38%), followed by cellular blue nevus (seven cases; 29%), and common blue nevus (six cases; 25%). Blue nevus/neurocristic hamartoma (one case; 4%) was less common. In one case in our series, melanoma arose in association with an atypical cellular blue nevus (4%). Identifying a benign nevus component in lesions like these is an important clinical variable (for staging and subsequent management), as an associated benign nevus comprises strong evidence for a primary lesion, which otherwise might be in debate for a predominantly dermal/subcutaneous-based malignant melanocytic proliferation without an obvious intraepidermal component/radial growth phase. In our experience, one helpful clue that is suggestive of an associated blue nevus component is the presence of a ‘dumbbell-shaped’ architecture to the melanoma.
An important limitation to our study was the fact that we designed our search for cases such that we specifically captured lesions with an unequivocally benign blue nevus and/or cellular blue nevus component. Therefore, the conclusions drawn from this study may only pertain to lesions with a distinctive benign blue nevus component and thus may not be as applicable to melanomas whose architecture resembles cellular blue nevi but without evidence of a true benign component. In contrast, most of the cases included in one of the larger studies available in the literature by Granter et al5 consisted of ‘malignant cellular blue nevi’ without a true benign nevus component. This may in part explain some of the differences observed by Granter et al5 in comparison with our findings, including differences in observed survival. In particular, all of their patients for whom follow-up information was available either developed local recurrence or metastasis or died of disease, while five (21%) of the patients in our series had no documented evidence of regional or distant metastasis and were alive at the time we concluded our study. Similarly, necrosis was a relatively common feature observed in our cases (46%) while only 20% of their cases demonstrated necrosis.
When we grouped the lesions according to the type of blue nevus component associated with the melanoma (common blue nevus, Supplementary Figure 1A; cellular blue nevus, Supplementary Figure 1B and those lesions resembling a cellular blue nevus, Supplementary Figure 1C), several interesting observations emerged. First, melanomas resembling cellular blue nevus but lacking a true benign component exhibit a slightly higher median age (52 years) compared with those arising in association with or common or cellular blue nevi (46 years and 43 years, respectively). Second, these melanomas exhibit an anatomic predilection for the head and neck independently of their associated blue nevus component. Thus, the anatomic site typical of the respective benign blue nevus counterpart does not impact the favored anatomic site of the so called ‘malignant blue nevus’.
Here, we summarize the features of 24 melanomas arising in association with common or cellular blue nevi and correlated these clinical and histopathologic features with annotated clinical follow-up information. We demonstrate a significant association between the variable of tumor thickness (defined as the largest recorded tumor dimension or Breslow thickness when applicable) with both recurrence-free survival and reduced time to distant metastasis, while other prognostic indicators predictive of outcome in conventional cutaneous melanomas do not correlate with outcomes in these rare and unique melanomas. We advocate inclusion of this variable in our synoptic reporting scheme. Additional larger studies are warranted to confirm this observation.
References
Jadassohn-Tieche M . Über benigne melanome (‘chromatophorome’) der haut-‘blaue naevi’. Virchows Arch Pathol Anat Physiol Klin Med 1906;186:212–229.
Rodriguez HA, Ackerman LV . Cellular blue nevus. Clinicopathologic study of forty-five cases. Cancer 1968;21:393–405.
Zembowicz A, Phadke PA . Blue nevi and variants: an update. Arch Pathol Lab Med 2011;135:327–336.
Allen AC, Spitz S . Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer 1953;6:1–45.
Granter SR, McKee PH, Calonje E et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called 'malignant blue nevus’. Am J Surg Pathol 2001;25:316–323.
Magro CM, Crowson AN, Mihm MC . Unusual variants of malignant melanoma. Mod Pathol 2006;19 (Suppl 2):S41–S70.
Martin RC, Murali R, Scolyer RA et al. So-called ‘malignant blue nevus’: a clinicopathologic study of 23 patients. Cancer 2009;115:2949–2955.
Avidor I, Kessler E . ‘Atypical’ blue nevus-a benign variant of cellular blue nevus. Presentation of three cases. Dermatologica 1977;154:39–44.
Tran TA, Carlson JA, Basaca PC et al. Cellular blue nevus with atypia (atypical cellular blue nevus): a clinicopathologic study of nine cases. J Cutan Pathol 1998;25:252–258.
Barnhill RL, Argenyi Z, Berwick M et al. Atypical cellular blue nevi (cellular blue nevi with atypical features): lack of consensus for diagnosis and distinction from cellular blue nevi and malignant melanoma (‘malignant blue nevus’). Am J Surg Pathol 2008;32:36–44.
Aloi F, Pich A, Pippione M . Malignant cellular blue nevus: a clinicopathological study of 6 cases. Dermatology 1996;192:36–40.
Ariyanayagam-Baksh SM, Baksh FK, Finkelstein SD et al. Malignant blue nevus: a case report and molecular analysis. Am J Dermatopathol 2003;25:21–27.
Boi S, Barbareschi M, Vigl E et al. Malignant blue nevus. Report of four new cases and review of the literature. Histol Histopathol 1991;6:427–434.
Boni R, Panizzon R, Huch Boni RA et al. Malignant blue naevus with distant subcutaneous metastasis. Clin Exp Dermatol 1996;21:427–430.
Calista D, Schianchi S, Landi C . Malignant blue nevus of the scalp. Int J Dermatol 1998;37:126–127.
Connelly J, Smith JL Jr. . Malignant blue nevus. Cancer 1991;67:2653–2657.
Dorsey CS, Montgomery H . Blue nevus and its distinction from Mongolian spot and the nevus of Ota. J Invest Dermatol 1954;22:225–236.
Duteille F, Duport G, Larregue M et al. Malignant blue nevus: three new cases and a review of the literature. Ann Plast Surg 1998;41:674–678.
Fisher ER . Malignant blue nevus. AMA Arch Derm 1956;74:227–231.
Gartmann H, Lischka G . [Malignant blue nevus. (Malignant dermal melanocytoma)]. Hautarzt 1972;23:175–178.
Goldenhersh MA, Savin RC, Barnhill RL et al. Malignant blue nevus. Case report and literature review. J Am Acad Dermatol 1988;19:712–722.
Hagiwara T, Kaku T, Kobayashi H et al. Coexisting vulvar malignant melanoma and blue nevus of the cervix. Gynecol Oncol 2005;99:519–520.
Held L, Metzler G, Eigentler TK et al. Recurrent nodules in a periauricular plaque-type blue nevus with fatal outcome. J Cutan Pathol 2012;39:1088–1093.
Hendrickson MR, Ross JC . Neoplasms arising in congenital giant nevi: morphologic study of seven cases and a review of the literature. Am J Surg Pathol 1981;5:109–135.
Hernandez FJ . Malignant blue nevus. A light and electron microscopic study. Arch Dermatol 1973;107:741–744.
Hourihane DO . A malignant blue naevus, with metastasis to regional lymph node. Ir J Med Sci 1971;140:169–175.
Hu W, Nelson JE, Mohney CA et al. Malignant melanoma arising in a pregnant African American woman with a congenital blue nevus. Dermatol Surg 2004;30:1530–1532.
Kato N, Tamura A, Yamanaka Y et al. Malignant blue nevus: case report of a Japanese man with a distant cutaneous metastasis. Am J Dermatopathol 2007;29:88–91.
Kuhn A, Groth W, Gartmann H et al. Malignant blue nevus with metastases to the lung. Am J Dermatopathol 1988;10:436–441.
Kwittken J, Negri L . Malignant blue nevus. Case report of a Negro woman. Arch Dermatol 1966;94:64–69.
Lee HY, Na SY, Son YM et al. A malignant melanoma associated with a blue nevus of the lip. Ann Dermatol 2010;22:119–124.
Lobo AZ, Martin RM, Belda W Jr et al. Disseminated blue naevus and malignant blue naevus associated with excessive aromatase syndrome. Clin Exp Dermatol 2008;33:591–594.
Mehregan DA, Gibson LE, Mehregan AH . Malignant blue nevus: a report of eight cases. J Dermatol Sci 1992;4:185–192.
Mellone P, Bianchi A, Dragonetti E et al. Malignant melanoma associated with a blue naevus: a case report. Cases J 2008;1:433.
Merkow LP, Burt RC, Hayeslip DW et al. A cellular and malignant blue nevus: a light and electron microscopic study. Cancer 1969;24:888–896.
Mishima Y . Cellular blue nevus. Melanogenic activity and malignant transformation. Arch Dermatol 1970;101:104–110.
Modly C, Wood C, Horn T . Metastatic malignant melanoma arising from a common blue nevus in a patient with subacute cutaneous lupus erythematosus. Dermatologica 1989;178:171–175.
North JP, Yeh I, McCalmont TH et al. Melanoma ex blue nevus: two cases resembling large plaque-type blue nevus with subcutaneous cellular nodules. J Cutan Pathol 2012;39:1094–1099.
Odashiro AN, Arthurs B, Pereira PR et al. Primary orbital melanoma associated with a blue nevus. Ophthal Plast Reconstr Surg 2005;21:247–248.
Ozgur F, Akyurek M, Kayikcioglu A et al. Metastatic malignant blue nevus: a case report. Ann Plast Surg 1997;39:411–415.
Parada D, Pena KB, Riu F . Coexisting malignant melanoma and blue nevus of the uterine cervix: an unusual combination. Case Rep Pathol 2012;2012:986542.
Pathy AL, Helm TN, Elston D et al. Malignant melanoma arising in a blue nevus with features of pilar neurocristic hamartoma. J Cutan Pathol 1993;20:459–464.
Pozo L, Diaz-Cano SJ . Malignant deep sclerosing blue naevus presenting as a subcutaneous soft tissue mass. Br J Dermatol 2004;151:508–511.
Rubinstein N, Kopolovic J, Wexler MR et al. Malignant blue nevus. J Dermatol Surg Oncol 1985;11:921–923.
Sanada S, Higaki K, Torii Y et al. Malignant melanoma arising in a plaque-type blue nevus. Pathol Int 2012;62:749–753.
Schneider S, Bartels CG, Maza S et al. Detection of micrometastasis in a sentinel lymph node of a patient with malignant blue nevus: a case report. Dermatol Surg 2006;32:1089–1092.
Scott GA, Trepeta R . Clear cell sarcoma of tendons and aponeuroses and malignant blue nevus arising in prepubescent children. Report of two cases and review of the literature. Am J Dermatopathol 1993;15:139–145.
Silverberg GD, Kadin ME, Dorfman RF et al. Invasion of the brain by a cellular blue nevus of the scalp. A case report with light and electron microscopic studies. Cancer 1971;27:349–355.
Spatz A, Zimmermann U, Bachollet B et al. Malignant blue nevus of the vulva with late ovarian metastasis. Am J Dermatopathol 1998;20:408–412.
Temple-Camp CR, Saxe N, King H . Benign and malignant cellular blue nevus. A clinicopathological study of 30 cases. Am J Dermatopathol 1988;10:289–296.
Wetherington GM, Norins AL, Sadove AM . Locally invasive cellular blue nevus of the scalp. Plast Reconstr Surg 1987;79:114–117.
Yeh I, Fang Y, Busam KJ . Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol 2012;36:1258–1263.
Zyrek-Betts J, Micale M, Lineen A et al. Malignant blue nevus with lymph node metastases. J Cutan Pathol 2008;35:651–657.
English JC 3rd, McCollough ML, Grabski WJ . A pigmented scalp nodule: malignant blue nevus. Cutis 1996;58:40–42.
Singh RR, Patel KP, Routbort MJ et al. Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. J Mol Diagn 2013;15:607–622.
Verma S, Greaves WO, Ravandi F et al. Rapid detection and quantitation of BRAF mutations in hairy cell leukemia using a sensitive pyrosequencing assay. Am J Clin Pathol 2012;138:153–156.
Balch CM, Gershenwald JE, Soong SJ et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199–6206.
Balch CM . Cutaneous melanoma: prognosis and treatment results worldwide. Semin Surg Oncol 1992;8:400–414.
Balch CM, Soong SJ, Gershenwald JE et al. Prognostic factors analysis of 17600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622–3634.
Barnhill RL, Katzen J, Spatz A et al. The importance of mitotic rate as a prognostic factor for localized cutaneous melanoma. J Cutan Pathol 2005;32:268–273.
Ostmeier H, Fuchs B, Otto F et al. Can immunohistochemical markers and mitotic rate improve prognostic precision in patients with primary melanoma? Cancer 1999;85:2391–2399.
Winnepenninckx V, Lazar V, Michiels S et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst 2006;98:472–482.
Acknowledgements
The authors would like to thank Ms Kim-Anh T Vu for her assistance with graphic design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Rights and permissions
About this article
Cite this article
Loghavi, S., Curry, J., Torres-Cabala, C. et al. Melanoma arising in association with blue nevus: a clinical and pathologic study of 24 cases and comprehensive review of the literature. Mod Pathol 27, 1468–1478 (2014). https://doi.org/10.1038/modpathol.2014.62
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2014.62
Keywords
This article is cited by
-
Primary orbital melanoma arising in an atypical diffuse (plaque-like) blue naevus/melanocytosis: a case report and review of literature
BMC Ophthalmology (2021)
-
Activating cysteinyl leukotriene receptor 2 (CYSLTR2) mutations in blue nevi
Modern Pathology (2017)
-
SF3B1 and BAP1 mutations in blue nevus-like melanoma
Modern Pathology (2017)
-
Genomic copy number analysis of a spectrum of blue nevi identifies recurrent aberrations of entire chromosomal arms in melanoma ex blue nevus
Modern Pathology (2016)
-
Molecular alterations in malignant blue nevi and related blue lesions
Virchows Archiv (2015)