Abstract
Serous ovarian cancer is suggested to develop from epithelium embryologically derived from the Müllerian ducts. The aim of the current study is to thoroughly, analyze the epithelium derived from the Müllerian ducts (cervix, endometrium and fallopian tubes) in serous ovarian cancer patients. Sixty women diagnosed with serous ovarian carcinoma were included in this multicentre, observational study. Tissues were embedded completely for histological assessment, in accordance with the SEE-Fim and SEE-End protocol (Sectioning and Extensively Examining of the Fimbriated end; and—Endometrium), and prevalence of cervical, as well as endometrial and tubal pathology was analyzed. In 31 (52%) cases, a pathologic lesion was identified, and in 16 (27%) of these cases coexistence of pathologic lesions. In 1 case, severe dysplasia was found in the cervix, in 9 (15%) cases endometrial intraepithelial carcinoma, in 19 (32%) cases atypical hyperplasia, and in 23 (43%) cases serous tubal intraepithelial carcinoma. Serous tubal intraepithelial carcinoma was seen significantly more often concurrent with endometrial atypical hyperplasia or endometrial intraepithelial carcinoma than with benign endometrium (64 vs 28%; P=0.01). To conclude, histological assessment of epithelium derived from Müllerian ducts of serous ovarian cancer patients resulted in the identification of endometrial intraepithelial carcinoma, serous tubal intraepithelial carcinoma and/or endometrial atypical hyperplasia in more than half of cases. Coexistence of these pathologic lesions was common, and might represent an effect of field carcinogenesis or tumor implantation of migrating cells.
Similar content being viewed by others
Main
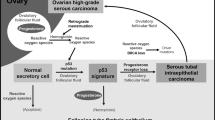
Epithelial ovarian cancer has the highest mortality rate of gynecologic malignancies. It was diagnosed with an incidence of 1400 women in The Netherlands in 2010, whereas 1065 women died from this disease.1, 2 The vast majority of epithelial ovarian cancers are high-grade serous carcinomas, accounting for 70% of cases. High-grade serous carcinomas are mostly diagnosed in an advanced stage of disease due to non-specific symptoms and a tendency for early peritoneal spread.3 The overall survival of patients with ovarian carcinoma has improved only minimally, and one of the reasons is that its route of development has not been totally clarified yet.
It is thought that ovarian carcinoma develops from tissues that are embryologically derived from the Müllerian ducts and that the ovaries are involved secondarily.4, 5 Müllerian ducts develop in early embryogenesis into the upper part of the vagina, uterus, and fallopian tubes.5 In the tubes the in situ carcinoma, serous tubal intraepithelial carcinoma, has been identified.6, 7 Serous tubal intraepithelial carcinoma is found in 41% of patients diagnosed with serous ovarian carcinoma.6, 8, 9, 10 However, in a significant part of serous ovarian cancer patients, no serous tubal intraepithelial carcinoma or predisposition for serous carcinoma has been found. Recently, endometrial intraepithelial carcinoma, was suggested as an alternative source for serous ovarian cancers, but its prevalence in ovarian cancer patients is unknown.11
The standard surgical treatment of serous ovarian carcinomas includes bilateral salpingo-oophorectomy and hysterectomy. The most important reason to perform a hysterectomy is that the uterine serosa is one of the most frequent sites of peritoneal spread.12 Moreover, double primary endometrial carcinomas are identified in 10% of endometrioid ovarian carcinomas and in 2–5% of other ovarian carcinoma types.13, 14, 15
The current study was performed to analyze the cervix, endometrium and tubal epithelium thoroughly in serous ovarian cancer patients. Therefore, structural embedding of the entire endometrium and fallopian tubal epithelium was performed and extensive histological assessment of tissue for all cases.
Materials and methods
Study Design and Case Selection
In this prospective, multicentre, cohort study, all women with high-grade serous ovarian cancer undergoing a primary or interval debulking surgery that included hysterectomy and bilateral salpingo-oophorectomy were identified. Women were operated at the Radboud University Nijmegen Medical Centre and Canisius Wilhelmina Hospital, Nijmegen, from September 2010 to December 2012. Surgery was performed by a gynecologic oncologist. Clinical parameters, such as age, menopausal status, body mass index, BRCA mutation status, treatment protocol, and medical history, were retrieved from medical records and pathology reports. Patients were classified and staged in accordance with the WHO classification and FIGO surgical staging adopted in 1988.16, 17 Carcinomas were classified as primary ovarian if the largest tumor bulk was located in the ovaries. In case of simultaneous carcinoma involvement of the endometrium, additionally depth of myometrial invasion, ovarian tumor pattern, lymfovascular invasion and presence of serous tubal intraepithelial carcinoma or endometrial intraepithelial carcinoma were used to differentiate primary ovarian from endometrial carcinoma.18
Histological Examination of Tissues Derived from the Müllerian Duct: Endocervix, Endometrium and Fallopian Tubes
All tissues were macroscopically assessed and described before formalin fixation overnight. The anterior side of the uterus was once cut several centimeters in longitudinal direction, from cervix toward fundus, to enable optimal fixation. The uterus was sampled using a systematic protocol with complete embedding of the endometrium, the SEE-End (Sectioning and Extensively Examining of the Endometrium) protocol (Figure 1). First of all, the uterus was cut longitudinally into two equal parts. Routine sections of the cervix uteri anterior and posterior were embedded, followed by a section of the isthmus region. A representative section of the fundus region was cut in longitudinal direction. Remaining endometrium was sectioned in transverse direction at intervals of 2–3 mm. For practical reasons, redundant myometrium was excised from additionally sampled sections to enable embedding of multiple endometrial samples in one paraffin block (Figure 1). In case of an endometrial polyp, it was embedded in toto. Representative sections were embedded from polyps of the cervix or isthmus, leiomyomas, or other lesions for histological examination.
The entire endometrium was sampled and embedded following the SEE-End protocol (Sectioning and Extensively Examining the Endometrium). After sectioning the standard diagnostic samples, the remaining endometrium was sectioned in transverse direction with intervals of 2–3 mm. Redundant myometrium was removed from additionally sampled endometrium to enable the display of several endometrial sections in one slide. (a) Cervix uteri anterior and posterior, (b) Isthmus, (c) representative section of fundus region of the endometrium, including an endometrial polyp. (d) Representative slide of small leiomyoma, (e–h) remaining endometrium and endometrial polyp were embedded in accordance with the SEE-End protocol.
The fallopian tubes were completely embedded for histological examination, in accordance with the SEE-Fim protocol (Sectioning and Extensively Examining of the Fimbriated end).19 The fimbriated end was amputated at the infundibulum and cut longitudinally in four sections to maximize exposure and optimize histological view of the tubal plicae. The fallopian tube was cross-sectioned at intervals of 2–3 mm.19
All histological sections were stained with hematoxylin and eosin and reviewed by two gyneco-pathologists. In case of discrepancies, consensus was reached between pathologists. The columnar epithelium of the cervix was examined for atypia, presence of adenocarcinoma in situ, or carcinoma. The nature of the endometrium was classified in the following categories: atrophic endometrium, proliferative, secretory, disordered proliferative, hyperplasia, atypical hyperplasia, endometrial intraepithelial carcinoma, or invasive carcinoma.
The classification system for endometrial hyperplasia outlined by the World Health Organization (WHO) in 2003 was used in the current study.17, 18 Hyperplasia was defined as proliferating endometrium with architectural abnormalities such as budding and branching. Cells are mostly enlarged and exhibit nuclear pseudostratification, with an increased number of mitotic figures. An increased gland-to-stroma ratio (3:1) is required for diagnosis of hyperplasia, and it can present with or without atypical cells.20 In case of proliferative features, in absence of increased gland-to-stroma ratio, the endometrium was diagnosed as proliferative. The endometrium of postmenopausal women with proliferative features was considered disordered proliferative. Endometrial intraepithelial carcinoma was proposed by Ambros et al,21 and described as noninvasive, serous carcinoma resembling cells, that replace the endometrial surface epithelium and glands. An example of atypical hyperplasia and endometrial intraepithelial carcinoma is shown in Figure 2.
(a) An example of serous carcinoma located in an ovary (level of magnification: × 100). The following pathologic lesions were identified in this cohort of women with serous ovarian carcinoma (level of magnification: × 100). (b) Serous tubal intraepithelial carcinoma located in the tubal epithelium, with adjacent benign tubal epithelium. (c) Endometrial intraepithelial carcinoma. (d) Atypical hyperplasia located in endometrial glands.
The tubal sections were classified as follows: normal tubal epithelium, hyperplasia, minor epithelial atypia, serous tubal intraepithelial carcinoma, or invasive carcinoma.22 Tubal hyperplasia was defined as cellular crowding in absence of atypical nuclei, and minor epithelial atypia as cellular crowding, with slight cellular atypia and nuclei with small nucleoli, but without loss of polarity. Serous tubal intraepithelial carcinoma is defined as noninvasive, serous carcinoma resembling cells that replace the tubal epithelium.23, 24, 25 They are characterized by a high nuclear-to-cytoplasm ratio, nuclear pleomorphism, stratification, and a high mitotic index. Further, the presence of serous tubal intraepithelial carcinoma in the fimbrial part of the fallopian tube, the non-fimbrial part, or in both locations was recorded.
Histological Examination of the Ovaries
The ovaries were embedded following standard protocol and sections were scored for histological carcinoma type, the location and extent of the tumor, and the tumor differentiation grade (low or high grade).26, 27 Furthermore, concurrent borderline tumors in the ovaries or other abnormalities were scored.
Immunohistochemistry
Immunohistochemical staining was performed for all cases identified with an endometrial intraepithelial carcinoma, and coexisting serous tubal intraepithelial carcinoma and/or atypical hyperplasia. Representative sections of 4 μm were sliced from formalin-fixed paraffin-embedded samples and incubated with monoclonal antibodies against p53 (dilution 1:250; Clone DO-7; Neomarkers, Fremont, CA, USA), Ki-67 (dilution 1:100; Clone MIB-1; Dako, Glostrup, Denmark), and WT1 (dilution 1:20; Clone WT49, NCL-L-WT1-562, Leica Biosystems, Newcastle Upon Tyne, UK). Antigen retrieval was processed according to the manufacturers’ guidelines. Immunohistochemical stainings were interpreted by two of the authors (JB, AAGvT). Staining was scored as the percentage of positive nuclear tumor cells and classified as follows: 0%, <5%, 5–25%, 26–50%, 51–75%, >75%. Intensity of staining was scored as weak, moderate or strong.
Statistical Analysis
Statistical analyses were performed using SPSS software version 20.0 (SPSS, Chicago, IL, USA) and P<0.05 (two-sided test) was considered statistically significant. For comparison of groups of women with or without serous tubal and/or endometrial intraepithelial carcinoma, the Pearson’s chi-square (χ2) test was used, or when appropriate, the Fisher’s exact test. For comparison of continuous variables between both groups, the Mann-Whitney U test was used.
Results
Clinical Characteristics
The study cohort included 60 women diagnosed with high-grade serous ovarian cancer. Clinical characteristics of these women are shown in Table 1. Mean age was 63 years, the majority were postmenopausal (88%) and mean body mass index was 24 kg/m2. The FIGO stage distribution was as follows: 3 (5%) women had a FIGO stage I, 4 (7%) a stage II, 47 (80%) a stage III, and 5 (8%) a stage IV. Seventy-five percent of patients received chemotherapy before debulking surgery.
Eleven (18%) patients had another cancer diagnosed before their diagnosis of ovarian carcinoma: eight were diagnosed with breast carcinoma, two with colon carcinoma and one with melanoma. In four cases, the breast carcinoma was diagnosed within 6 months from the ovarian carcinoma, and they were considered double primary carcinomas. In four (7%) cases of the study population, a BRCA1 mutation was identified, but analysis was not performed for all included women. Three BRCA1 mutation carriers also had a diagnosis of breast carcinoma.
Overview of Tissues Derived from the Müllerian duct in Patients with Serous Ovarian Cancer
In three cases, the cervix was not available for histological examination, because a supravaginal hysterectomy was performed at debulking surgery. No pathology could be found in the endocervix of women with serous ovarian carcinoma, except for one case with serous carcinoma expanding toward the cervix, and another case with severe dysplasia (CIN 3).
In Table 2, results are shown of the histological examination of the endometrial sections. The endometrium was diagnosed as benign endometrium in 38 (63%) cases: in 29 (48%) cases the endometrium was atrophic, (disordered) proliferative or secretory, and in 9 cases (15%) hyperplastic. In 13 (22%) cases the endometrium showed atypical hyperplasia, in 3 (5%) cases an endometrial intraepithelial carcinoma was identified, and in 6 (10%) endometrial intraepithelial carcinoma and atypical hyperplasia. In total, endometrial intraepithelial carcinoma was found in 9 (15%) cases and atypical hyperplasia in 19 cases (32%).
Concurrent invasive serous endometrial carcinoma was found in five cases, and all occurred in cases diagnosed with additionally endometrial intraepithelial carcinoma and/or atypical hyperplasia. Invasive endometrial carcinoma was present in 23% (5/22) of cases diagnosed with endometrial intraepithelial carcinoma and/or atypical hyperplasia. All invasive endometrial carcinomas were of serous type. In one case it was seen concurrent to atypical hyperplasia, in one case concurrent to endometrial intraepithelial carcinoma, and in three cases concurrent to both endometrial intraepithelial carcinoma and atypical hyperplasia. In one of the latter, the endometrial and ovarian carcinoma were interpreted as double primaries, with the largest primary carcinoma located in the endometrium and a second focus of carcinoma located adjacent to borderline carcinoma in the left ovary. Four cases were classified as primary ovarian carcinoma with metastasis in the endometrium, as the largest carcinoma mass was located in the ovary and diffuse ovarian involvement was seen.
Results of histological assessment of the tubal epithelium was shown in Table 3. In 18 (33%) cases the tubal epithelium was normal, in 2 (4%) cases hyperplasia was found, in 11 (20%) cases minor epithelial atypia, and in 23 (43%) cases serous tubal intraepithelial carcinoma was identified. Serous tubal intraepithelial carcinoma was identified in the fimbrial region of the fallopian tubes in 15 (65%) cases, in the non-fimbrial region in 4 (17%) cases, and in both fimbrial and non-fimbrial regions in 4 (17%) cases. In six (8%) cases the tubal epithelium was not available for histological assessment, in four cases the tubes could not be identified in the tumor mass, and in two cases the tubes were already removed during incomplete debulking surgery in another hospital.
In 50% (30/60) of cases with serous ovarian carcinoma, also invasive carcinoma was found in the tubal epithelium. The carcinoma was restricted to the fimbrial part of the tube in 28% (17/60) of cases. In 60% (18/30) of cases with serous invasive tubal carcinoma, additionally a serous intraepithelial carcinoma was found in the tubal epithelium, whereas serous tubal intraepithelial carcinoma was found in only 17% (5/30) of cases without serous tubal carcinoma.
Characteristics of Serous Ovarian Carcinoma with Endometrial Intraepithelial Carcinoma and/or Serous Tubal Intraepithelial Carcinoma
Clinical and pathological characteristics were compared between groups of women diagnosed with endometrial intraepithelial carcinoma and/or serous tubal intraepithelial carcinoma, and women diagnosed without these intraepithelial carcinomas (Table 4). None of the characteristics were significantly different between the two groups of women, except that the diagnosis of cancer before the ovarian carcinoma was more common in cases diagnosed with serous tubal and/or endometrial intraepithelial carcinomas (P=0.004). Cases who underwent chemotherapy before debulking surgery did not show significantly more often serous tubal and/or endometrial intraepithelial carcinoma (P=0.872). Further, bilateral ovarian carcinoma involvement, enlarged ovaries, presence of additional ovarian lesions as cysts, or type of ovarian carcinoma pattern (diffuse/multinodular/mainly surface) was not significantly different for cases diagnosed with or without serous tubal intraepithelial carcinoma and/or endometrial intraepithelial carcinoma.
Coexisting Endometrial Intraepithelial Carcinoma and Serous Tubal Intraepithelial Carcinoma in Müllerian-Derived Tissues of Serous Ovarian Cancer Patients
In Table 5, an overview is given of the histologically examined tissue of all cases in which a serous tubal intraepithelial carcinoma, endometrial intraepithelial carcinoma, and/or atypical hyperplasia was found concurrent to serous ovarian carcinoma. In 31 (52%) cases one or more of these lesions were identified in the analyzed tissues, in 9 (15%) cases serous tubal intraepithelial carcinoma was found, in 5 (8%) atypical hyperplasia, in 1 (2%) endometrial intraepithelial carcinoma, and in 16 (27%) cases multiple pathological lesions were found. In eight (13%) cases serous tubal intraepithelial carcinoma and atypical hyperplasia, in five (8%) cases endometrial intraepithelial carcinoma, as well as atypical hyperplasia and serous tubal intraepithelial carcinoma, in two (3%) cases endometrial intraepithelial carcinoma and atypical hyperplasia, and in one (2%) case serous tubal and endometrial intraepithelial carcinoma (Table 5).
Immunophenotypes of endometrial intraepithelial carcinoma were compared with phenotypes of coexisting serous tubal intraepithelial carcinoma and/or atypical hyperplasia (Table 6). Six cases analyzed with serous tubal intraepithelial carcinoma and endometrial intraepithelial carcinoma, showed almost comparable p53 as well as Ki-67 expression in coexisting lesions. WT1 showed a difference in expression of more than one expression class in three cases, and was comparable in two. In one of these cases, tissue of the serous tubal intraepithelial carcinoma was not available for additional incubation with monoclonal antibody against WT1. Immunophenotypes of coexisting endometrial intraepithelial carcinoma and atypical hyperplasia showed lower expression for p53 and Ki-67 in atypical hyperplasia compared with endometrial intraepithelial carcinoma, and WT1 was negative in all atypical hyperplasia, regardless of the WT1 expression in the endometrial intraepithelial carcinoma.
Serous Tubal Intraepithelial Carcinoma, Endometrial Intraepithelial Carcinoma and/or Atypical Hyperplasia Occur Significantly more often Synchronously
In Table 7, the prevalence of serous tubal intraepithelial carcinoma is analyzed in concurrence to the different entities identified in the endometrium. Serous tubal intraepithelial carcinoma was significantly more often found in concurrence to endometrial intraepithelial carcinoma, atypical hyperplasia, and/or invasive endometrial carcinoma compared with normal endometrium (P=0.010; Pearson chi-square). In 64% of cases identified with serous tubal intraepithelial carcinoma, an endometrial intraepithelial carcinoma and/or atypical hyperplasia was found in the endometrium, whereas in 28% of cases with serous tubal intraepithelial carcinoma the endometrium was benign. Of the latter, in four cases non-atypical hyperplasia was found in the endometrium.
Discussion
High-grade serous ovarian carcinoma is suggested to originate from tissues embryologically derived from the Müllerian ducts, followed by spread into the abdominal cavity by exfoliation and migration of loose cohesive cells.23, 28 The fallopian tubes have been extensively examined in the last decade and serous tubal intraepithelial carcinoma has been found in serous ovarian carcinomas in a mean overall prevalence of 41%.6, 8, 9, 10 The current study is unique, as the entire epithelium of the endometrium and the tubal epithelium were extensively assessed, as well as representative sections of the cervix. In 52% of cases, a serous tubal intraepithelial carcinoma, endometrial intraepithelial carcinoma, and/or endometrial atypical hyperplasia was found. In the endocervical tissue, one CIN3 lesion was found. In the fallopian tubes, serous tubal intraepithelial carcinoma was present in 43%, and in the endometrium an intraepithelial carcinoma was found in 15% of cases and atypical hyperplasia in 32%. In a quarter of cases, a lesion was found in the tubes as well as in the endometrium. Serous tubal intraepithelial carcinoma occurred significantly more often in women diagnosed with an endometrial intraepithelial carcinoma and/or atypical hyperplasia compared with women with a benign endometrium, regardless of the type of endometrial lesion (P=0.010).
To the best of our knowledge, the surprisingly high prevalence of atypical hyperplasia and endometrial intraepithelial carcinoma in serous ovarian cancer patients has not been previously described. The few studies that investigated the endometrium in pathology reports of asymptomatic women, reported a much lower prevalence of atypical hyperplasia (0.5–1.1%) and no endometrial intraepithelial carcinoma.29, 30, 31 We recently reported the prevalence of atypical hyperplasia in 6% of women who underwent a hysterectomy because of uterovaginal prolapse, after embedding the endometrial tissue in accordance with the SEE-End protocol.32 In none of these women, endometrial intraepithelial carcinoma was found.
The prevalence of serous tubal intraepithelial carcinoma in the current study was comparable to other studies that extensively examined the fallopian tubes in accordance with the SEE-Fim protocol.6, 9 In these studies, serous tubal intraepithelial carcinoma, was found restrictive to ovarian cancers of serous histology type, which supports its suggested role in the development of serous carcinoma.10, 33 Tubal hyperplasia and minor epithelial atypia were equally present in women at risk of ovarian carcinoma compared with women from normal populations, and considered as variants of normal tubal epithelial proliferation.22
Recently, the introduction of the SEE-Fim protocol resulted in a significant increase of the identification of serous tubal intraepithelial carcinoma, as it enabled optimal microscopic view of the tubal plicae.19 The endometrium is not particularly difficult to visualize microscopically, but its heterogeneous aspect and the small size of some lesions necessitates meticulous screening.20 The introduction of the SEE-End protocol might contribute to a more accurate identification of endometrial pathology, as shown by the higher prevalence of endometrial pathology found in asymptomatic women after introduction of the new protocol.32 Especially for the identification of endometrial intraepithelial carcinoma, the SEE-End protocol is recommended, as it can occur only unifocal and can be easily missed with routine sampling.
Further, identification of invasive endometrial carcinoma seems slightly increased in the current study (8%), compared with the prevalence reported after routine sampling (2–5%).15, 34 This might be because of the introduction of the SEE-End protocol. In half of the cases, additional serous tubal carcinoma was found and in one case was found even in the endocervix. Prevalence of extensive carcinoma is high and we cannot exclude that some of these cases might be misclassified as primary ovarian instead of primary tubal or endometrial. However, diagnostic criteria for extensive serous carcinoma have not been well defined.
The high prevalence of endometrial atypical hyperplasia in this group of serous ovarian cancer patients is remarkable, as atypical hyperplasia is known as a precursor of endometrioid endometrial carcinoma and its development is influenced by genetic predisposition and increased, unopposed, estrogen.35 Some endometrioid ovarian cancers have been described to cause increased estrogen levels, and this has even been reported for a few cases with serous ovarian cancer.36, 37, 38 However, we have no information on estrogen levels from cases included in the current study.
Endometrial intraepithelial carcinoma is involved in the development of serous endometrial carcinoma, and is occasionally found concurrent to serous ovarian or extra-ovarian carcinoma, without presence of invasive endometrial carcinoma.11, 39, 40, 41 An endometrial intraepithelial carcinoma has the capability of disseminating throughout the peritoneal cavity, and could putatively be responsible for a proportion of serous epithelial ovarian cancers.42, 43
Interestingly, we often identified simultaneous prevalence of serous tubal intraepithelial carcinoma, endometrial intraepithelial carcinoma, and/or atypical hyperplasia in patients with serous ovarian carcinoma. Several hypotheses can be put forward to explain their coexistence. Endometrial atypical hyperplasia represents most likely a synchronous precursor of endometrioid endometrial carcinoma in these women with serous ovarian carcinoma, instead of a lesion involved in the development of serous ovarian cancer. This is supported by the different immunophenotypes for atypical hyperplasia and coexisting endometrial intraepithelial carcinoma. However, serous tubal intraepithelial carcinoma and endometrial intraepithelial carcinoma are both involved in the development of serous carcinoma, and their coexistence is in contrast with the assumption that serous carcinoma develops from one location, from where it can develop into invasive carcinoma or spread to other locations in the peritoneal cavity.
An explanation for coexisting serous tubal and endometrial intraepithelial carcinomas is that one lesion represents the primary location from where the tumor spreads, and that the other lesion is a direct product of implantation of migrating cells. Spread of tumor cells from an endometrial intraepithelial carcinoma into the uterine cavity, through the tubes, toward the peritoneal cavity seems a plausible explanation, as it follows the route of retrograde menstruation.11, 43, 44 However, as suggested by Jarboe et al,40 serous tubal intraepithelial carcinoma might also represent the primary location, as cells may exfoliate toward the peritoneal cavity as well as toward the uterine cavity.45 They reported on a few cases diagnosed as endometrial serous carcinoma with coexistence of serous tubal intraepithelial carcinoma and endometrial intraepithelial carcinoma.40 However, most cases were without invasive endometrial carcinoma, but with tumor involvement of both ovaries. Therefore, cases seem comparable to the ones diagnosed in the current study as serous ovarian cancer with coexisting serous tubal and endometrial intraepithelial carcinomas. Jarboe et al,40 analyzed the tumor origin of four cases with coexisting intraepithelial carcinomas with immunohistochemical Wilms tumor 1 staining. Results were inconclusive and comparable to ours, as two cases showed strong expression in both lesions, whereas one case showed heterogeneous expression and another one none.40
Another intriguing explanation for the coexistence of serous tubal intraepithelial carcinoma and endometrial intraepithelial carcinoma is that carcinoma development does not occur in only one specific area of the Müllerian duct, but that the entire tract is susceptible for tumor development in these women. This could explain for the discordant WT1 staining in some of the coexisting serous tubal and endometrial intraepithelial carcinomas. A similar mechanism of field carcinogenesis has been described in many cancers, as for example in colonic and head and neck cancers, and has occasionally been described for ovarian cancers.46, 47, 48 This mechanism could also explain that gynecologic carcinomas, of comparable or different histological subtypes, are often identified synchronously. Additional molecular genetic studies are necessary to differentiate primary carcinomas from metastases and to elucidate the route of carcinogenesis in these women.
Clinical and pathological characteristics were almost comparable for women with and without a serous tubal and/or endometrial intraepithelial carcinoma in the current study. However, clinical variables were retrieved retrospectively and not always complete. The majority of women included in this study underwent chemotherapy before debulking surgery. Although, no significant difference in prevalence of endometrial intraepithelial carcinoma, serous tubal intraepithelial carcinoma or atypical hyperplasia was found between both groups, we cannot exclude that chemotherapy might have an impact on the prevalence of pathologic lesions. Another limitation of the current study is that the cervical tissue was not completely embedded.
In conclusion, the current study provided an overview of pathology in tissues derived from the Müllerian duct in women with serous ovarian cancer, embedded in accordance with the SEE-Fim and SEE-End protocol. Atypical hyperplasia, serous tubal intraepithelial carcinoma, and endometrial intraepithelial carcinoma were commonly identified in these women, and often occurred simultaneously. The clinical significance and biological potential of these lesions in women diagnosed with serous ovarian carcinoma may be of utmost importance in understanding tumor development of ovarian cancers.
References
van Altena AM, Karim-Kos HE, de Vries E et al. Trends in therapy and survival of advanced stage epithelial ovarian cancer patients in the Netherlands. Gynecol Oncol 2012;125:649–654.
Registration WNC. www.cijfersoverkanker.nl(IKNL; Integraal Kankercentrum Nederland) 2013.
Gross AL, Kurman RJ, Vang R et al. Precursor lesions of high-grade serous ovarian carcinoma: morphological and molecular characteristics. J Oncol 2010;2010:126295.
Marquez RT, Baggerly KA, Patterson AP et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res 2005;11:6116–6126.
Dubeau L . The cell of origin of ovarian epithelial tumours. Lancet Oncol 2008;9:1191–1197.
Kindelberger DW, Lee Y, Miron A et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol 2007;31:161–169.
Piek JM, van Diest PJ, Zweemer RP et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol 2001;195:451–456.
Przybycin CG, Kurman RJ, Ronnett BM et al. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol 2010;34:1407–1416.
Roh MH, Kindelberger D, Crum CP . Serous tubal intraepithelial carcinoma and the dominant ovarian mass: clues to serous tumor origin? Am J Surg Pathol 2009;33:376–383.
Tang S, Onuma K, Deb P et al. Frequency of serous tubal intraepithelial carcinoma in various gynecologic malignancies: a study of 300 consecutive cases. Int J Gynecol Pathol 2012;31:103–110.
Roelofsen T, van Kempen LC, van der Laak JA et al. Concurrent endometrial intraepithelial carcinoma (EIC) and serous ovarian cancer: can EIC be seen as the precursor lesion? Int J Gynecol Cancer 2012;22:457–464.
Bafghi A, Zafrani Y, Pautier P et al. Endometrial disorders in patients with peritoneal serous papillary carcinoma. Eur J Obstet Gynecol Reprod Biol 2007;134:101–104.
Silverman BB, O'Neill RT, Mikuta JJ . Multiple malignant tumors associated with primary carcinoma of the ovary. Surg Gynecol Obstet 1972;134:243–248.
Soliman PT, Slomovitz BM, Broaddus RR et al. Synchronous primary cancers of the endometrium and ovary: a single institution review of 84 cases. Gynecol Oncol 2004;94:456–462.
van Altena AM, Geels YP, Bulten J et al. Why do women with double primary carcinoma of the endometrium and ovary have a favorable prognosis? Int J Gynecol Pathol 2012;31:344–351.
Heintz AP, Odicino F, Maisonneuve P et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95 (Suppl 1):S161–S192.
Lee KRTF, Tavassoli FA, (eds) World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press: Lyon, 2003.
McCluggage WG . Benign diseases of the endometrium In: Kurman RJ, Ellenson HL (eds) Blaustein's Pathology of the Female Genital Tract 6th edn. Springer: New York, 2011, pp 305–358.
Medeiros F, Muto MG, Lee Y et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol 2006;30:230–236.
Mills AM, Longacre TA . Endometrial hyperplasia. Semin Diagn Pathol 2010;27:199–214.
Ambros RA, Sherman ME, Zahn CM et al. Endometrial intraepithelial carcinoma: a distinctive lesion specifically associated with tumors displaying serous differentiation. Hum Pathol 1995;26:1260–1267.
Mingels MJ, Roelofsen T, van der Laak JA et al. Tubal epithelial lesions in salpingo-oophorectomy specimens of BRCA-mutation carriers and controls. Gynecol Oncol 2012;127:88–93.
Crum CP, Drapkin R, Miron A et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol 2007;19:3–9.
Piek JM, Verheijen RH, Kenemans P et al. BRCA1/2-related ovarian cancers are of tubal origin: a hypothesis. Gynecol Oncol 2003;90:491.
Mehrad M, Ning G, Chen EY et al. A pathologist's road map to benign, precancerous, and malignant intraepithelial proliferations in the fallopian tube. Adv Anat Pathol 2010;17:293–302.
Kommoss S, Schmidt D, Kommoss F et al. Histological grading in a large series of advanced stage ovarian carcinomas by three widely used grading systems: consistent lack of prognostic significance. A translational research subprotocol of a prospective randomized phase III study (AGO-OVAR 3 protocol). Virchows Arch 2009;454:249–256.
Malpica A, Deavers MT, Lu K et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol 2004;28:496–504.
Finch A, Beiner M, Lubinski J et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. JAMA 2006;296:185–192.
Bonnar J, Kraszewski A, Davis WB . Incidental pathology at vaginal hysterectomy for genital prolapse. J Obstet Gynaecol Br Commonw 1970;77:1137–1139.
Frick AC, Walters MD, Larkin KS, Barber MD . Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol 2010;202:507–4.
Mahajan G, Kotru M, Batra M et al. Usefulness of histopathological examination in uterine prolapse specimens. Aust NZ J Obstet Gynaecol 2011;51:403–405.
Mingels MJ, Geels PG, Pijnenborg JM et al. Histopathologic assessment of the entire endometrium in asymptomatic women. Human Pathol 2013;44:2293–2301.
Jarboe E, Folkins A, Nucci MR et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol 2008;27:1–9.
Bunting MW, Jaaback KS, McNally OM . Routine hysterectomy in the surgical management of ovarian cancer: a retrospective case series, physician opinion survey, and review of the literature. Int J Gynecol Cancer 2011;21:1579–1584.
Kurman RJ, McConnell TG . Precursors of endometrial and ovarian carcinoma. Virchows Arch 2010;456:1–12.
Jongen VH, Sluijmer AV, Heineman MJ . The postmenopausal ovary as an androgen-producing gland; hypothesis on the etiology of endometrial cancer. Maturitas 2002;43:77–85.
Poels LG, Jap PH, Ramaekers FF et al. Characterization of a hormone-producing ovarian carcinoma cell line. Gynecol Oncol 1989;32:203–214.
Ridderheim M, Mahlck CG, Selstam G et al. Steroid production in different parts of malignant and benign ovarian tumors in vitro. Cancer Res 1993;53:2309–2312.
Baergen RN, Warren CD, Isacson C et al. Early uterine serous carcinoma: clonal origin of extrauterine disease. Int J Gynecol Pathol 2001;20:214–219.
Jarboe EA, Miron A, Carlson JW et al. Coexisting intraepithelial serous carcinomas of the endometrium and fallopian tube: frequency and potential significance. Int J Gynecol Pathol 2009;28:308–315.
Soslow RA, Pirog E, Isacson C . Endometrial intraepithelial carcinoma with associated peritoneal carcinomatosis. Am J Surg Pathol 2000;24:726–732.
Zheng W, Schwartz PE . Serous EIC as an early form of uterine papillary serous carcinoma: recent progress in understanding its pathogenesis and current opinions regarding pathologic and clinical management. Gynecol Oncol 2005;96:579–582.
Massuger L, Roelofsen T, Ham M et al. The origin of serous ovarian cancer may be found in the uterus: a novel hypothesis. Med Hypotheses 2010;74:859–861.
Kurman RJ, Shih IeM . The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 2010;34:433–443.
Seidman JD, Zhao P, Yemelyanova A . "Primary peritoneal" high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol Oncol 2011;120:470–473.
Slaughter DP, Southwick HW, Smejkal W . Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953;6:963–968.
Buller RE, Skilling JS, Sood AK et al. Field cancerization: why late "recurrent" ovarian cancer is not recurrent. Am J Obstet Gynecol 1998;178:641–649.
Damania D, Roy HK, Kunte D et al. Insights into the field carcinogenesis of ovarian cancer based on the nanocytology of endocervical and endometrial epithelial cells. Int J Cancer 2013;133:1143–1152.
Acknowledgements
This work was funded by Ruby and Rose Foundation. This is an original study that has not been submitted for publication elsewhere.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mingels, M., van Ham, M., de Kievit, I. et al. Müllerian precursor lesions in serous ovarian cancer patients: using the SEE-Fim and SEE-End protocol. Mod Pathol 27, 1002–1013 (2014). https://doi.org/10.1038/modpathol.2013.212
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.212
Keywords
This article is cited by
-
Perceptions of risk and reward in BRCA1 and BRCA2 mutation carriers choosing salpingectomy for ovarian cancer prevention
Familial Cancer (2020)
-
Evaluation of SEE-FIM (Sectioning and Extensively Examining the FIMbriated End) Protocol in Identifying Fallopian Tube Precursor Lesions in Women with Ovarian Tumors
The Journal of Obstetrics and Gynecology of India (2019)
-
Müllerian intra-abdominal carcinomatosis in hereditary breast ovarian cancer syndrome: implications for risk-reducing surgery
Familial Cancer (2016)
-
Prophylactic bilateral salpingectomy (PBS) to reduce ovarian cancer risk incorporated in standard premenopausal hysterectomy: complications and re-operation rate
Journal of Cancer Research and Clinical Oncology (2014)