Abstract
The biological behavior of teratomas is highly variable, and morphologic features alone are insufficient to predict their clinical course. Prognostic factors that influence behavior include the following: patient sex, age, anatomic site, coincident neoplasm, and cytogenetic abnormalities. Gonadal teratomas have been well-characterized; postpubertal testicular teratomas are commonly associated with isochromosome 12p (i12p) and considered to nearly always carry a potential for malignant behavior, whereas ovarian and prepubertal testicular teratomas are i12p negative and predominantly benign in behavior. For extragonadal sites, such as sacrum and coccyx, clinical characteristics and i12p status are yet to be adequately characterized. As part of this study, we identified 19 sacrococcygeal teratomas in our surgical pathology archives from 1990 to 2012. Clinical records and slides were reviewed to confirm the original diagnosis. Gains in chromosome 12p, including i12p status were assessed in representative paraffin sections by fluorescence in situ hybridization. Our cases included 16 mature sacrococcygeal teratomas (11 prepubertal and 5 postpubertal) and three immature saccrococygeal teratomas (all prepubertal). Among mature teratomas, the average tumor size was larger in adults compared with prepubertal patients. A higher number of adult cases were recurrences (80% vs 21%), but only pediatric recurrences were managed with postoperative chemotherapy. All examined tumors were negative for i12p. 100% survival was documented in our cohort with a median follow-up of 6 years. We present a large series of sacrococcygeal teratomas and the first series to examine postpubertal adults at this anatomic site. All tumors lacked chromosome 12p gains, including i12p. Both pre- and postpubertal sacrococcygeal teratomas had a favorable outcome regardless of age or sex.
Similar content being viewed by others
Main
Teratomas are diverse neoplasms that may represent a challenge for diagnosis, prognosis, and treatment. Teratomas by definition display histologic components derived from at least two of three developmental germ layers and are classified as a subgroup within germ cell tumors. Germ cell tumors arise primarily within the gonads, but a significant minority can also be found in extragonadal midline locations, including the sacrum, presacral space, coccyx, mediastinum, retroperitoneum, and pineal/intracranial space.1 The biological behavior and prognosis of extragonadal teratomas have been interpreted analogously to the tumors in the ovaries and testes, using criteria such as age, size, the presence of immature elements, and concurrent somatic malignant transformation of any of its elements. Whereas ovarian teratomas are typically cystic, composed of mature elements and almost uniformly benign, postpubertal testicular tumors are considered to nearly always2 carry a potential for malignant behavior, even when composed entirely of mature elements.3 The cytogenetic and molecular characteristics that underpin the differences among gonadal germ cell tumors are now better understood, with the landmark finding of isochromosome 12p or 12p amplification in the majority of testicular germ cell tumors,4 but a complete absence thereof in benign, mature ovarian teratomas.5 A recent study of anterior mediastinal extragonadal germ cell tumors has shown that the biological behavior and prognosis of teratomas at this site is intermediate between those of the testis and the ovary.6 Studies addressing other extragonadal sites are still lacking, particularly in adults.
Here we review the clinicopathologic characteristics and the i12p status for a large series of primary sacrococcygeal teratomas from our institution.
Materials and methods
Criteria for Selection of Patients
The study was approved by the institutional review board at our hospital. The surgical pathology database at the Johns Hopkins Hospital (Baltimore, MD, USA) was searched for all in-house teratoma cases occurring in the sacrum and coccyx between 1990 and 2012. All microscopic slides were re-evaluated by a senior urological pathologist on the study (GJN). Medical records were examined for clinical and laboratory data, including tumor size (based on available gross pathologic examination or radiologic assesssment when the latter was not recorded), alpha fetoprotein, adjuvant chemotherapy, and recurrence and survival data. A total of 22 cases were identified. One case was excluded based on a change of diagnosis to epidermoid inclusion cyst upon review. Another case in the series contained focal yolk sac elements/endodermal sinus tumor, and upon retrospective review an additional case (reported as a mature teratoma) was found to represent a recurrence; to maintain a clear, focused study cohort, both were excluded from further analysis. Blocks were available in 16 of the 19 cases in the current study.
Fluorescence in Situ Hybridization
Interphase FISH was performed on all 16 tumors where formalin-fixed and paraffin-embedded blocks were available, using a 12 centromere control probe (D12Z3, Abbott Molecular-Vysis, Des Plaines, IL, USA) and a probe for 12p12.1 previously validated in our laboratory for clinical identification of i12p.7 i12p is an abnormal chromosome formed by a transverse rather than normal longitudinal splitting of a replicating chromosome, resulting in a median centromere with two identical arms of 12p. Briefly, the centromeric probe was labeled with green fluorescence, whereas the 12p probe is labeled with Orange-dUTP fluorescence (02N33-050, Abbott Molecular). The slides were deparaffinized using a VP 2000 processor (Abbott Molecular). Following deparaffinization the slides and the probe mix were co-denatured at 80 °C for 7 min and allowed to hybridize over night at 37 °C in humidified atmosphere. At the end of the incubation the slides were washed in 2 X SSC/0.3% NP-40 for 2 min at 72 °C and for 2 min at room temperature. The slides were counterstained with DAPI and a cover slip was applied using Vectashield mounting medium (H-1000, Vector Laboratories, Inc.). The signal pattern was evaluated in 60 cells within the area designated on the H&E slide and expressed as a ratio, with 12p/centromere ratio above 1.5 judged as indicative of i12p. FISH was successful in 13 of 16 cases, with no additional results upon repeat hybridization. Among three cases not successfully hybridized, two were more than 10 years old (initial surgical material from 1990 to 2000) and one was from an extremely scant biopsy specimen, with only minimal material remaining upon reprocessing.
Statistical Analysis
Significance of tumor size was evaluated using a non-parametric analysis of variance (Kruskall–Wallis). Significance for categorical variables (recurrence rates, chemotherapy, elevated AFP, and survival and i12p status) was evaluated using a χ2-test. In all statistical evaluations, P ≤ 0.05 was considered statistically significant.
Results
A total of 19 cases were included in the study. Clinicopathologic characteristics are shown in Table 1 and summarized in Table 2. Fourteen cases were prepubertal and five cases were postpubertal; 16 cases consisted entirely of mature teratomatous tissues and 3 showed at least focal immature component hereafter referred to as immature; the adult patients ranged from 32 to 51 years with a median age of 45, whereas the pediatric patients ranged from neonates (2 days old) to a 4-year-old with a median age of 3 months; available follow-up was variable, but generally longer in pediatric patients than in adult patients (8.3 vs 1.2 years); there was a female predominance in both groups, particularly within the small subset of sacrococcygeal teratomas containing immature elements.
Tumor characteristics were compared between adult and pediatric tumors (Table 3). For mature teratomas, the average tumor size was significantly larger in adults than in prepubertal patients (9.1 vs 5.4 cm; P=0.018). Among prepubertal patients, immature teratomas were highly variable but generally larger than mature counterparts (11.3 vs 5.4 cm, not significant). On the basis of the available clinical history, 3 of the 14 pediatric cases were recurrences; among adults, 4 out of 5 cases were recurrences of previously resected pediatric tumors. The fifth adult teratoma was detected incidentally without a previously established pediatric tumor.
All adult patients were treated by surgical excision alone. A subset of older (>12 months) pediatric patients, particularly those with elevated α-fetoprotein, were treated with both surgical excision and chemotherapy. The tumor marker α-fetoprotein (AFP) was measured in 10 of 14 pediatric patients, and adjusting for gestational age8 was found to be elevated in two. AFP was not available in any of the adult cases. No fatalities were attributed to the tumors, and all patients presented in this case series are still alive.
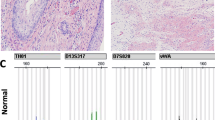
We also investigated the isochromosome 12p status among both pediatric and adult sacrococcygeal teratomas. None of the cases we successfully tested were positive for i12p, including the immature sacrococcygeal teratomas (Figure 1 and Table 3). The only incidental finding was the detection of focal increase in 12p signal within a carcinoid arising within a mature teratoma portion of that case (case 3, Figures 1e and f). Upon further inspection, there was a coincident increase in the centromeric signal, such that 12p/centromere ratio was ∼1 and did not represent i12p. Altogether, no differences in the ratio of the targeted 12p/centromere signal were detected in multiple comparisons, including males vs females, adults vs pediatric patient, and primary tumors vs recurrences.
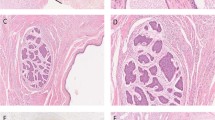
(a, b) Case 9, representative mature teratoma: (a) Hematoxylin-eosin/H&E stain ( × 400 magnification) of mature, stratified ciliated epithelium; (b) negative chromosome 12p FISH, selected field within the boxed area. (c, d) Case 2, immature teratoma: (c) H&E ( × 400 magnification) of immature neuronal tissue. (d) Negative chromosome 12p FISH, selected field within the boxed area. (e, f) Case 3, carcinoid arising within a mature elements of the teratoma: (e) H&E ( × 400 magnification) carcinoid arising in a mature teratoma; (f) Chromosome 12p FISH, selected field within the boxed area; arrows highlight a focal increase in both green-red probes without increase in their ratio (approximately 1:1), among several cells within the carcinoid.
Discussion
Teratomas are diverse neoplasms with a wide range of epidemiology, histological characteristics, and biological behavior. The prognostic implications of a teratoma diagnosis have been primarily based on three factors: the anatomic location of the tumor, patient demographics, and the presence of coincident malignancy (teratoma with somatic malignant transformation) or immature histological elements. Ovarian teratomas are nearly uniformly benign regardless of age and show only moderately increased risk with the presence of immature elements.9 In the testes, however, teratomas tend to be benign in prepubertal males yet harbor increased risk of malignant behavior in postpubertal patients regardless of histology.9 In addition, testicular teratomas are known to harbor higher rates of concurrent somatic malignant transformation and/or other germ cell tumors, which necessitate wide sampling and careful follow-up. Among extragonadal sites, a large case series and meta-analysis have shown mature teratomas of anterior mediastinum to be largely but not uniformly benign, whereas teratomas with immature elements have a substantial risk of aggressive behavior (metastasis or recurrence).6 This places teratomas of the anterior mediastinum as a prognostically intermediate group between teratomas of the testes and those of the ovary. The data are largely lacking for other anatomic sites including the retroperitoneum,10 sacrum and coccyx, particularly in adults; perhaps questionably, clinical decisions are currently made based on extrapolations from what is known about gonadal teratomas or a handful of case reports.
Sacrococcygeal teratomas are most commonly seen as congenital neoplasms with an incidence of ∼1:35 000–40 000 of live births.11 These tumors are much less common in adults and are thought to represent either a previously undetected or an incompletely excised congenital tumor.12 Only limited studies examining sacrococcygeal teratomas for genetic abnormalities have been performed, and almost none in adults. One case series failed to detect any abnormal karyotypes in five sacrococcygeal pediatric immature teratomas.13 Another case series of pediatric teratomas did not identify any recurrent abnormalities, and all sacrococcygeal teratomas showed a normal karyotype.3 Remaining studies are largely individual case reports and demonstrate similar findings.14, 15, 16, 17, 18 For adults, only one case of an untreated congenital sacrococcygeal teratoma has been examined in detail: this tumor underwent a malignant transformation to an adenocarcinoma, with cytogenetic aberrations (amplifications of 8q and 12p) present only within the adenocarcinoma component.19 The remaining literature consists of purely descriptive individual case reports.
Our case series represents the largest report to date of adult sacrococcygeal teratomas. We confirm that adult sacrococcygeal teratomas mostly (80%) represent known recurrences, which suggests that even incidentally discovered adult mature sacrococcygeal teratomas may have been previously unrecognized or untreated tumors. The pathological tumor characteristics in our study are well-defined and include tumor size, margin status, and the explicit exclusion of any tumor with other malignant histological elements (other germ cell tumor elements, like yolk sac tumor or concurrent testicular germ cell tumors in males). Many tumors (10/21) were entirely submitted, with the remaining sampled exhaustively. We correlate these data with clinical characteristics such as age, tumors markers (alpha fetoprotein/AFP), initial imaging and importantly follow-up data such as social security index-linked mortality, time to recurrence (if any), and postoperative tumor marker measurements. Our data strongly support the conclusion that resected mature sacrococcygeal teratomas in pediatric patients, even those with subsequent recurrences, will ultimately have a favorable outcome20 and suggest that recurrent sacrococcygeal teratomas in postpubertal patients are also indolent.
The genetic aberrations that underpin teratomas are thought to be quite diverse. The best studied among them is i12p.4 This cytogenetic abnormality is present in the majority of testicular teratomas, regardless of age and presence of immature elements. In contrast, i12p is largely absent in immature ovarian teratomas and uniformly absent in mature ones. The i12p status at extragonadal sites remains poorly understood. In the largest study of the pediatric population via genome-wide, array-based comparative genomic hybridization,21 seven out of eight mature sacrococcygeal teratomas and one of two immature sacrococcygeal teratomas showed no cytogenetic aberrations; the two outlier cases were both negative for i12p or gain of 12p. Aside from the above mentioned single case report of mature teratoma with an element of adenocarcinoma,19 the cytogenetic profile of adult sacrococcygeal teratomas has never been examined. Here, we evaluate i12p status of sacrococcygeal teratoma using FISH. In the cases we were able to examine, the i12p aberration was absent regardless of age, gender, or subsequent recurrence, including one case of immature sacrococcygeal teratoma. i12p status may also prove to be a useful diagnostic and potentially prognostic marker, particularly in males. In a large series (n=143) of postpubertal men with germ cell tumors, most primary tumors (90%) were in the testes and most nonseminomatous germ cell tumors (79%) were positive for i12p.22 Although low case numbers precluded a definite analysis, patients with i12p appeared to do poorly with higher likelihood of incomplete response to therapy, recurrence or death than the counterparts without the chromosomal aberration. The authors of that work and other studies23, 24 indicate the presence of i12p as a hallmark finding diagnostic of midline germ cell tumors and i12p positivity as a factor in carcinogenesis, including the evolving understanding of the stroma23 and invasive growth.24 In addition, testicular teratomas can metastize to the sacrum and retroperitoneum, whereas primary testicular sites can regress or involute. Thus far, the recommended management of germ cell tumors from the presacral space and retroperitoneum in males involves a testicular ultrasound with or without biopsy and a lengthy follow-up. Given the odds at each anatomic site further highlighted by our study, positivity for i12p in extragonadal tumors may prompt consideration of repeat search for a testicular primary.
Here we review the clinicopathologic characteristics and the i12p status for a large series of primary sacrococcygeal teratomas from our institution. We show that mature sacrococcygeal teratomas behave in a benign manner. Like ovarian teratomas, we show that sacrococcygeal teratomas are negative for alterations in chromosome 12p, although a larger series may warrant examination. Within our subset of immature sacrococcygeal teratomas in neonates, all had a favorable outcome following a surgical resection.
References
Harms D, Zahn S, Gobel U et al. Pathology and molecular biology of teratomas in childhood and adolescence. Klin Padiatr 2006;218:296–302.
Zhang C, Berney DM, Hirsch MS et al. Evidence supporting the existence of benign teratomas of the postpubertal testis: a clinical, histopathologic, and molecular genetic analysis of 25 cases. Am J Surg Pathol 2013;37:827–835.
Bussey KJ, Lawce HJ, Olson SB et al. Chromosome abnormalities of eighty-one pediatric germ cell tumors: sex-, age-, site-, and histopathology-related differences-a Children's Cancer Group study. Genes Chromosomes Cancer 1999;25:134–146.
Mostert MC, Verkerk AJ, van de Pol M et al. Identification of the critical region of 12p over-representation in testicular germ cell tumors of adolescents and adults. Oncogene 1998;16:2617–2627.
Poulos C, Cheng L, Zhang S et al. Analysis of ovarian teratomas for isochromosome 12p: evidence supporting a dual histogenetic pathway for teratomatous elements. Mod Pathol 2006;19:766–771.
Yalcin B, Demir HA, Tanyel FC et al. Mediastinal germ cell tumors in childhood. Pediatr Hematol Oncol 2012;29:633–642.
Wehle D, Yonescu R, Long PP et al. Fluorescence in situ hybridization of 12p in germ cell tumors using a bacterial artificial chromosome clone 12p probe on paraffin-embedded tissue: clinical test validation. Cancer Genet Cytogenet 2008;183:99–104.
Blohm ME, Vesterling-Horner D, Calaminus G et al. Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr Hematol Oncol 1998;15:135–142.
Ulbright TM . Gonadal teratomas: a review and speculation. Adv Anat Pathol 2004;11:10–23.
Gatcombe HG, Assikis V, Kooby D et al. Primary retroperitoneal teratomas: a review of the literature. J Surg Oncol 2004;86:107–113.
Schropp KP, Lobe TE, Rao B et al. Sacrococcygeal teratoma: the experience of four decades. J Pediatr Surg 1992;27:1075–1078.
Ng EW, Porcu P, Loehrer PJ Sr . Sacrococcygeal teratoma in adults: case reports and a review of the literature. Cancer 1999;86:1198–1202.
Hoffner L, Deka R, Chakravarti A et al. Cytogenetics and origins of pediatric germ cell tumors. Cancer Genet Cytogenet 1994;74:54–58.
Le Caignec C, Winer N, Boceno M et al. Prenatal diagnosis of sacrococcygeal teratoma with constitutional partial monosomy 7q/trisomy 2p. Prenat Diagn 2003;23:981–984.
Noguera R, Navarro S, Carda C et al. Near-haploidy in a malignant sacrococcygeal teratoma. Cancer Genet Cytogenet 1999;108:70–74.
Veltman I, van Asseldonk M, Schepens M et al. A novel case of infantile sacral teratoma and a constitutional t(12;15)(q13;q25) pat. Cancer Genet Cytogenet 2002;136:17–22.
Veltman IM, Schepens MT, Looijenga LH et al. Germ cell tumours in neonates and infants: a distinct subgroup? APMIS 2003;111:152–160.
Wax JR, Benn P, Steinfeld JD et al. Prenatally diagnosed sacrococcygeal teratoma: a unique expression of trisomy 1q. Cancer Genet Cytogenet 2000;117:84–86.
Golas MM, Gunawan B, Raab BW et al. Malignant transformation of an untreated congenital sacrococcygeal teratoma: a amplification at 8q and 12p detected by comparative genomic hybridization. Cancer Genet Cytogenet 2010;197:95–98.
Marina NM, Cushing B, Giller R et al. Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children's Cancer Group Intergroup Study. J Clin Oncol 1999;17:2137–2143.
Veltman I, Veltman J, Janssen I et al. Identification of recurrent chromosomal aberrations in germ cell tumors of neonates and infants using genomewide array-based comparative genomic hybridization. Genes Chromosomes Cancer 2005;43:367–376.
Bosl GJ, Ilson DH, Rodriguez E et al. Clinical relevance of the i(12p) marker chromosome in germ cell tumors. J Natl Cancer Inst 1994;86:349–355.
Cheng L, Zhang S, Eble JN et al. Molecular genetic evidence supporting the neoplastic nature of fibrous stroma in testicular teratoma. Mod Pathol 2012;25:1432–1438.
Rosenberg C, Van Gurp RJ, Geelen E et al. Overrepresentation of the short arm of chromosome 12 is related to invasive growth of human testicular seminomas and nonseminomas. Oncogene 2000;19:5858–5862.
Acknowledgements
This work was presented, in part, at the 102nd Annual Meeting of the United States and Canadian Academy of Pathology in Baltimore, MD in March 2013. This work was supported in part by the Brady Institute PATANA fund. We thank Dr Steven Rowe for feedback about follow-up management from radiological standpoint and Molly Van Appledorn for statistical analysis consultation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gurda, G., VandenBussche, C., Yonescu, R. et al. Sacrococcygeal teratomas: clinico-pathological characteristics and isochromosome 12p status. Mod Pathol 27, 562–568 (2014). https://doi.org/10.1038/modpathol.2013.171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.171
Keywords
This article is cited by
-
Meningioma in mature cystic teratoma of the ovary: clinical and computed tomography findings
Cancer Imaging (2020)
-
Adrenal Teratoma: a Case Series and Review of the Literature
Endocrine Pathology (2017)
-
Expression pattern of clinically relevant markers in paediatric germ cell- and sex-cord stromal tumours is similar to adult testicular tumours
Virchows Archiv (2014)