Abstract
Activating mutations in the TERT promoter leading to increased telomerase expression were recently identified in cutaneous melanoma and subsequently in many other types of cancer. These mutations lead to increased telomerase expression, allowing cells to proliferate continuously without entering apoptosis or senescence. Atypical fibroxanthomas and pleomorphic dermal sarcomas are genetically poorly understood tumors developing in the skin of older patients. Known genetic events in these tumors are mutations in TP53 (atypical fibroxanthoma and pleomorphic dermal sarcoma) and RAS (pleomorphic dermal sarcoma) genes, often having a UV signature. We analyzed a cohort of 27 atypical fibroxanthomas and 34 pleomorphic dermal sarcomas for the presence of TERT promoter mutations by conventional Sanger sequencing. TERT promoter mutations were identified in 25 (93%) atypical fibroxanthomas and in 26 (76%) pleomorphic dermal sarcomas. Mutations were found to have a UV signature (C>T or CC>TT) and were largely identical to those detected in cutaneous melanoma. Our data show that TERT promoter mutations are the most frequent mutations in atypical fibroxanthomas and pleomorphic dermal sarcomas reported to date. The identified mutations confirm the pathogenetic role of UV exposure in both atypical fibroxanthomas and pleomorphic dermal sarcomas and suggest that telomere maintenance through increased expression of telomerase plays an important role in the pathogenesis of these tumors.
Similar content being viewed by others
Main
Atypical fibroxanthomas and pleomorphic dermal sarcomas of the skin are rare mesenchymal tumors that typically arise in the skin of older patients and are genetically poorly characterized.
Atypical fibroxanthomas are rapidly growing exophytic tumors that occur in sun-damaged skin, primarily in the head and neck region of elderly patients.1, 2, 3 They are well-circumscribed tumors occurring more frequently in males. Risk factors include UV exposure, irradiation, xeroderma pigmentosum, and organ transplantation.4 Histologically, they are usually composed of atypical spindled and pleomorphic tumor cells including tumor giant cells, and are centered within the dermis with limited extension into the subcutis. They show high mitotic activity including atypical mitotic figures.2 However, they do not invade into the deep soft tissues, and despite increased proliferative activity, areas of tumor necrosis and lymphovascular and/or perineural invasion are not present. A diagnosis of atypical fibroxanthoma requires exclusion of other neoplasms, in particular, melanoma, squamous cell carcinoma, and leiomyosacroma. Atypical fibroxanthomas generally have a good prognosis, and usually complete excision and regular follow-up are recommended.1
The term ‘pleomorphic dermal sarcoma’ was introduced by Fletcher5 and describes tumors presenting with a similar morphology to atypical fibroxanthomas, but which in addition show extensive involvement of subcutis and/or invasion into deeper structures, areas of tumor necrosis, lymphovascular invasion, and perineural invasion. Pleomorphic dermal sarcomas exhibit more aggressive clinical behavior than atypical fibroxanthomas, and have the potential for local recurrence and metastasis.6 They are therefore categorized as tumors with low-grade malignant potential.6 The tumors now defined as pleomorphic dermal sarcomas have also been referred to as cutaneous undifferentiated pleomorphic sarcomas, and in the past as superficial malignant fibrous histiocytomas.7, 8, 9 There are currently no effective therapies for metastasized pleomorphic dermal sarcomas.
Distinguishing atypical fibroxanthoma from pleomorphic dermal sarcoma is not possible based on cell morphology alone, as they may show similar cyotologic features. The criteria allowing the distinction of pleomorphic dermal sarcoma from atypical fibroxanthoma are: extensive infiltration of subcutis, or invasion into fascia or muscle; necrosis; and vascular or perineural invasion.2, 5 Making the distinction between these tumors based solely on biopsy specimens should be avoided, as biopsies can be superficial or not show the deepest extent of tumor involvement. Although attempts have been made to identify immunohistochemical markers facilitating the distinction of atypical fibroxanthoma from pleomorphic dermal sarcoma (eg CD99 and LN-2 (refs. 10, 11)), none have proven reliable in routine practice.1, 2, 3
Little is known of the genetic events leading to the development of atypical fibroxanthomas and pleomorphic dermal sarcomas. Previous small studies identified UV-signature mutations in TP53 in atypical fibroxanthomas (7/10 (ref. 12) and 4/6 (ref. 13) cases) and pleomorphic dermal sarcomas (1/4 cases, diagnosed as ‘malignant fibrous histiocytomas’13), as well as one HRAS mutation and one KRAS mutation in eight pleomorphic dermal sarcomas (diagnosed as ‘malignant fibrous histiocytomas’) analyzed.14 In another study, pleomorphic dermal sarcomas (diagnosed as ‘undifferentiated pleomorphic sarcomas’) were found to harbor more frequent DNA copy number alterations than atypical fibroxanthomas.15
Two recent studies in cutaneous melanomas identified novel recurrent mutations in the promoter region of TERT, coding for the catalytic subunit of the telomerase holoenzyme, in up to 71% of tumors.16, 17 The mutations showed a characteristic UV signature (C>T and CC>TT).18 Functional studies found that these mutations lead to increased gene expression, most likely by creating ETS transcription factor binding sites.16, 17 A follow-up study screening a panel of different neoplasms identified TERT promoter mutations in a number of other common cancers, that is, in high frequencies in hepatocellular cancer, bladder cancer, and gliomas.19
In view of (a) the common occurrence of TERT promoter mutations with a UV signature in melanomas, (b) the strong association of atypical fibroxanthomas with UV exposure, and (c) the histologic similarities between atypical fibroxanthomas and pleomorphic dermal sarcomas, we investigated the presence of TERT promoter mutations in these tumors.
Materials and methods
Sample Selection
Atypical fibroxanthoma and pleomorphic dermal sarcoma tumor samples were obtained from the tissue archives of Dermatopathology Duisburg (17 atypical fibroxanthomas) and Dermatopathology Friedrichshafen (10 atypical fibroxanthomas, 9 pleomorphic dermal sarcomas), Germany, as well as from the Department of Pathology, Western General Hospital, The University of Edinburgh, Edinburgh, UK (25 pleomorphic dermal sarcomas, which were previously described by Miller et al6). The study was carried out in accordance with the guidelines set forth by the ethics committee of the University of Duisburg-Essen.
Histopathology and Immunohistochemistry
Histologic sections of all tumors were reviewed, and the diagnoses were confirmed by dermatopathologists (TB, TM, KGG, and JS). Analysis of available clinical and pathologic data was performed, including age, sex, site, size, depth, polyploid architecture, tumor mitotic rate, ulceration, necrosis, subcutis invasion, smooth muscle or fascia invasion, infiltrative or pushing border, lympovascular invasion, and perineural invasion. All tumors were stained with a panel of immunohistochemical markers including: pan-cytokeratin (MNF116; 1/500, AE1/AE3; 1/50), CD31 (JC70A; 1/100) or CD34 (QBEnd-10; 1/50), S100 (polyclonal; 1/2000), desmin (D33; 1/100), smooth muscle actin (ASMA 1A4; 1/500), and melan-A (A103; 1/1000). All antibodies were obtained from DAKO Hamburg/Germany (Duisburg and Friedrichshafen cases). Stains in the cases from Edinburgh were performed as described previously.6
DNA Isolation
Sections, 10-μm thick, were cut from formalin-fixed, paraffin-embedded tumor tissues. The sections were deparaffinized and tumor-bearing areas were manually macrodissected according to standard procedures. Genomic DNA was isolated using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Direct (Sanger) Sequencing
PCR amplification of a 474 bp region of the TERT promoter region was carried out applying the following primers: hTERT_F, 5′-ACGAACGTGGCCAGCGGCAG-3′ and hTERT_R, 5′-CTGGCGTCCCTGCACCCTGG-3′. In cases where amplification of the large fragment failed, primers hTERT_short_F, 5′-CAGCGCTGCCTGAAACTC-3′ and hTERT_short_R, 5′-GTCCTGCCCCTTCACCTT-3′, which amplify a 163 bp fragment, were applied as described previously.17 PCR products were purified with the QIAquick PCR Purification Kit (Qiagen) and used as templates for sequencing. The sequencing chromatogram files were examined and mutations were identified using Chromas software (version 2.01; University of Sussex, Brighton, UK) or Sequencher software (version 5.1; Gene Codes Corporation, Ann Arbor, MI, USA).
Results
Study Cohort
Sixty-one (27 atypical fibroxanthomas and 34 pleomorphic dermal sarcomas) tumors were analyzed, of which 59 were primary tumor samples and 2 were recurrent pleomorphic dermal sarcomas. Fifty-two patients were male and 9 were female. The median age at diagnosis was 82 (range 47–102) and 79 (range 65–93) years for patients with atypical fibroxanthomas, and 84 (range 47–102) years for patients with pleomorphic dermal sarcomas.
Clinical and Pathologic Features
Clinical and pathologic features are listed in Table 1. Representative images of an atypical fibroxanthoma and pleomorphic dermal sarcoma are shown in Figure 1. Tumors were generally negative for all listed immunohistochemical markers. Focal or patchy smooth muscle actin expression was seen in 11 (40%) atypical fibroxanthomas and 13 (39%) pleomorphic dermal sarcomas. As reported previously,6 11 (32%) pleomorphic dermal sarcomas showed focal expression of CD31 and 1 pleomorphic dermal sarcoma exhibited limited aberrant expression of melan-A (S100 was negative).
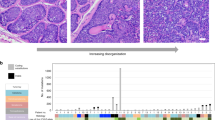
Examples of histologic features of atypical fibroxanthomas and pleomorphic dermal sarcomas. (a and b) Example of an atypical fibroxanthoma. (a) Relatively circumscribed, dermally centered tumor abutting the underlying subcutis. The overlying epidermis is thin and slightly invaginated (hematoxylin–eosin, × 20). (b) The tumor is composed of atypical spindled and pleomorphic tumor cells (hematoxylin–eosin, × 200). (c–e) Examples of pleomorphic dermal sarcomas. (c) Ulcerated, highly cellular tumor extensively infiltrating the dermis and subcutis (hematoxylin–eosin, × 20). (d) Another highly cellular tumor exhibiting areas of necrosis (hematoxylin–eosin, × 20). (e) High-power image of an area composed of highly atypical spindled and pleomorphic tumor cells and exhibiting areas of necrosis (hematoxylin–eosin, × 200).
TERT Promoter Mutation Analysis
Only samples showing unambiguous reads were included in the study. TERT promoter mutations were identified in 25 of 27 atypical fibroxanthomas (93%) and 26 of 34 pleomorphic dermal sarcomas (76%) (Table 2). Most of the mutations were identical to those described in cutaneous melanoma: chr. 5.1295228C>T (n=19, 31%); chr. 5.1295228_1295229 CC>TT (n=9, 15%); chr. 5.1295242_1295243CC>TT (n=5, 8%); and 1295250C>T (n=20, 33%). Mutations can alternatively be labeled according to their upstream location to the ATG initiation codon of TERT, which would be c.−124C>T, c. −124_125CC>TT, c. −138_139CC>TT, and c. −146C>T, respectively. For simplicity, we will refer to the mutations in the text using the last three digits of the chromosome location nomenclature, as 228C>T, 228CC>TT, 242CC>TT, and 250C>T, respectively (Figure 2).
TERT promoter mutations identified in atypical fibroxanthomas and pleomorphic dermal sarcomas. (a) Mutations identified at or around the chr. 5.1295228 location. (Mutations in the figure are abbreviated using only the last three digits of the location of the first nucleotide mutated.) (b) Mutations identified in the chr. 5.1295242 and chr. 5.1295250 location (both abbreviated as mentioned before). (c) Example of one case that was identified harboring mutations at all mentioned sites.
In addition to these frequent mutations, a single 228CCC>TTT (chr. 5.1295228_1295229_1295230CCC>TTT) mutation was identified. Three tumors harbored either concurrent 228C>T and 230C>T (atypical fibroxanthoma), 228C>T and C250>T (pleomorphic dermal sarcoma), or 250C>T and 253C>T (atypical fibroxanthoma) mutations, and one tumor showed concurrent 228CC>TT, 242CC>TT, and 250C>T mutations (pleomorphic dermal sarcoma, Figure 2). All identified TERT promoter mutations had a UV signature (C>T and CC>TT).18 Most mutations were heterozygous; however, homozygous mutations affecting both alleles were also found (Figure 2).
Associations of Clinical and Pathologic Parameters with TERT Promoter Mutation Status
We explored associations of TERT promoter mutation status with each of the clinical and pathologic parameters listed in Table 1. The only feature that showed a statistically significant association with mutation status was necrosis, which was present in 11/26 (42%) and 8/8 (100%) of mutant and wild-type pleomorphic dermal sarcomas, respectively (P=0.005).
Discussion
TERT promoter mutations appear to be common genetic events in human cancers. Originally found in melanoma, they were later identified in a large variety of other human cancers.19 Our findings indicate that they are also common in atypical fibroxanthomas and pleomorphic dermal sarcomas. The frequency of mutations (93% in atypical fibroxanthomas and 76% in pleomorphic dermal sarcomas) is even higher than those described in cutaneous melanoma (up to 71%).16, 17 TERT promoter mutations were frequent in tumors arising from tissues with low self-renewal.19 Although conclusive experimental evidence to support the validity of this hypothesis in atypical fibroxanthomas and pleomorphic dermal sarcomas is lacking, it would appear to also apply to these tumors, which have been postulated to derive from fibroblastic/myofibroblastic cells.1, 20, 21, 22, 23, 24, 25, 26
UV exposure is considered to be a major risk factor for atypical fibroxanthoma, but its link to pleomorphic dermal sarcoma is less clear.9, 12, 13, 14 In our cohort, the clinical presentation of both tumors was very similar, with tumors arising in elderly patients, on heavily sun-exposed areas (primarily head), with a strong male predominance (less hair protection), all of which support a role for UV exposure. The identification of TP53 mutations with a UV signature is supportive of a pathogenetic role of UV exposure.12, 13 All mutations identified in our study had a UV signature (C>T or CC>TT). However, both 228C>T and 250C>T mutations have also been found in tumor types for which a UV exposure link is unlikely, such as hepatocellular carcinoma and gliomas.19 In contrast, CC>TT mutations were not reported in the aforementioned tumors, but have been described in cutaneous melanoma.17 This is consistent with CC>TT mutations being virtually pathognomonic for UV exposure.18, 27 We found high percentages of CC>TT mutations not only in atypical fibroxanthomas but also in pleomorphic dermal sarcomas. Nine (33%) atypical fibroxanthomas had CC>TT mutations (six 228CC>TT, three 242CC>TT) and five (15%) pleomorphic dermal sarcomas harbored CC>TT mutations (three 228CC>TT, two 242CC>TT, one 228CCC>TTT—two of these found in one sample). These high mutation frequencies support UV exposure as an important pathogenetic factor in atypical fibroxanthoma and, to a lesser extent, pleomorphic dermal sarcoma.
Functional studies have shown that TERT promoter mutations lead to two- to fourfold increase in gene expression.16, 17 The suggested mechanism is through the introduction of ETS transcription binding sites.16, 17, 19 In the present study, the mutations were found almost exclusively in the previously described TERT promoter mutation hotspots (54 of 56 mutations identified in 51 tumors, with all tumors harboring at least one hotspot mutation), even though there are multiple adjacent nucleotides that could acquire UV-induced C>T or CC>TT mutations (Figure 2). Although supporting functional data is not available for our cohort, the localization of the mutation to hotspots implies a high selection pressure for the identified mutations, resulting in overexpression of the holoenzyme telomerase.16, 17 Telomerase expression in tumors is critical for maintaining telomere length and chromosomal stability, allowing tumor cells to go through continuous rounds of cell division without becoming genetically unstable, and avoiding apoptosis or senescence.28, 29
In certain cancer types, Killela et al19 described alternative lengthening of telomeres associated with DAXX and ATRX mutations as a mechanism for telomere maintenance in tumors that lacked TERT promoter mutations. To our knowledge, alternative lengthening of telomeres has not been analyzed in atypical fibroxanthomas or pleomorphic dermal sarcomas. Although our findings imply that increased TERT expression is the predominant mechanism for telomere maintenance in these tumors, we cannot exclude the possibility that alternative lengthening of telomeres may have a role in the few cases that lack TERT promoter mutations.
Considerable debate remains as to the relationship between atypical fibroxanthomas and pleomorphic dermal sarcomas. Atypical fibroxanthoma was long regarded as a superficial variant of pleomorphic dermal sarcoma (then diagnosed as malignant fibrous histiocytoma). The genetic data presented by Mihic-Probst et al15 suggests a pathogenetic link. They found that atypical fibroxanthomas and pleomorphic dermal sarcomas (for which they used the term ‘undifferentiated pleomorphic sarcomas’) shared many common copy number alterations, although higher numbers of alterations were detected in the latter.15 However, the relationship (if any exists) between the two tumors remains to be fully elucidated.
The genetic underpinnings of atypical fibroxanthoma and pleomorphic dermal sarcoma are poorly understood. To date, only TP53 mutations in 67–70%12, 13 of atypical fibroxanthomas and 25% of pleomorphic dermal sarcomas (diagnosed as superficial malignant fibrous histiocytomas13) and RAS mutations in 25% of pleomorphic dermal sarcomas (diagnosed as malignant fibrous histiocytoma14) have been described. The mutations in the TERT promoter described in the present study constitute the most prevalent mutation identified to date in both tumors.
In summary, our data highlight that TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas, and represent the most common mutation described in these tumors to date. The considerable number of CC>TT mutations found further suggests a role for UV induction in the pathogenesis of atypical fibroxanthomas and pleomorphic dermal sarcomas. Future studies will be required to determine whether TERT promoter mutation status could be of prognostic or therapeutic relevance.
References
Iorizzo LJ III, Brown MD . Atypical fibroxanthoma: a review of the literature. Dermatol Surg 2011;37:146–57.
Gru AA, Santa Cruz DJ. . Atypical fibroxanthoma: a selective review. Semin Diagn Pathol 2013;30:4–12.
McCalmont TH . AFX: what we now know. J Cutan Pathol 2011;38:853–6.
McCoppin HH, Christiansen D, Stasko T et al. Clinical spectrum of atypical fibroxanthoma and undifferentiated pleomorphic sarcoma in solid organ transplant recipients: a collective experience. Dermatol Surg 2012;38:230–9.
McCalmont TH . Correction and clarification regarding AFX and pleomorphic dermal sarcoma. J Cutan Pathol 2012;39:8.
Miller K, Goodlad JR, Brenn T . Pleomorphic dermal sarcoma: adverse histologic features predict aggressive behavior and allow distinction from atypical fibroxanthoma. Am J Surg Pathol 2012;36:1317–26.
Ozzello L, Stout AP, Murray MR . Cultural characteristics of malignant histiocytomas and fibrous xanthomas. Cancer 1963;16:331–44.
Fletcher CD . The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology 2006;48:3–12.
Henderson MT, Hollmig ST . Malignant fibrous histiocytoma: changing perceptions and management challenges. J Am Acad Dermatol 2012;67:1335–41.
Hartel PH, Jackson J, Ducatman BS, Zhang P . CD99 immunoreactivity in atypical fibroxanthoma and pleomorphic malignant fibrous histiocytoma: a useful diagnostic marker. J Cutan Pathol 2006;33 (Suppl 2):24–8.
Lazova R, Moynes R, May D, Scott G . LN-2 (CD74). A marker to distinguish atypical fibroxanthoma from malignant fibrous histiocytoma. Cancer 1997;79:2115–24.
Dei Tos AP, Maestro R, Doglioni C et al. Ultraviolet-induced p53 mutations in atypical fibroxanthoma. Am J Pathol 1994;145:11–7.
Sakamoto A, Oda Y, Itakura E et al. Immunoexpression of ultraviolet photoproducts and p53 mutation analysis in atypical fibroxanthoma and superficial malignant fibrous histiocytoma. Mod Pathol 2001;14:581–8.
Sakamoto A, Oda Y, Itakura E et al. H-, K-, and N-ras gene mutation in atypical fibroxanthoma and malignant fibrous histiocytoma. Hum Pathol 2001;32:1225–31.
Mihic-Probst D, Zhao J, Saremaslani P et al. CGH analysis shows genetic similarities and differences in atypical fibroxanthoma and undifferentiated high grade pleomorphic sarcoma. Anticancer Res 2004;24:19–26.
Huang FW, Hodis E, Xu MJ et al. Highly recurrent TERT promoter mutations in human melanoma. Science 2013;339:957–9.
Horn S, Figl A, Rachakonda PS et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013;339:959–61.
Pleasance ED, Cheetham RK, Stephens PJ et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010;463:191–6.
Killela PJ, Reitman ZJ, Jiao Y et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA 2013;110:6021–6.
Ito A, Yamada N, Yoshida Y, Morino S, Yamamoto O . Myofibroblastic differentiation in atypical fibroxanthomas occurring on sun-exposed skin and in a burn scar: an ultrastructural and immunohistochemical study. J Cutan Pathol 2011;38:670–6.
Beer TW, Drury P, Heenan PJ. . Atypical fibroxanthoma: a histological and immunohistochemical review of 171 cases. Am J Dermatopathol 2010;32:533–40.
Weedon D, Kerr JF . Atypical fibroxanthoma of skin: an electron microscope study. Pathology 1975;7:173–7.
Starink TH, Hausman R, Van Delden L, Neering H . Atypical fibroxanthoma of the skin. Presentation of 5 cases and a review of the literature. Br J Dermatol 1977;97:167–77.
de Feraudy S, Mar N, McCalmont TH . Evaluation of CD10 and procollagen 1 expression in atypical fibroxanthoma and dermatofibroma. Am J Surg Pathol 2008;32:1111–22.
Jensen K, Wilkinson B, Wines N, Kossard S . Procollagen 1 expression in atypical fibroxanthoma and other tumors. J Cutan Pathol 2004;31:57–61.
Alguacil-Garcia A, Unni KK, Goellner JR, Winkelmann RK . Atypical fibroxanthoma of the skin: an ultrastructural study of two cases. Cancer 1977;40:1471–80.
Brash DE, Rudolph JA, Simon JA et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA 1991;88:10124–8.
Blackburn EH . Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 2005;579:859–62.
Gunes C, Rudolph KL . The role of telomeres in stem cells and cancer. Cell 2013;152:390–3.
Acknowledgements
We thank Iris Moll and Mingxia Song for their excellent technical support. The presented study was supported by a grant from the Mercur-Stiftung. The funders were not involved in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DS is on the advisory board or has received honararia from Roche, Genetech, Novartis, Amgen, GSK, BMS, Boehringer Ingelheim, and Merck. LZ has received honoraria from Roche, Bristol-Meyers Squibb, and Amgen, and travel support from Merck Sharp & Dohme and Bristol-Meyers Squibb. BS received travel support from Bristol-Meyers Squibb. All the other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Griewank, K., Schilling, B., Murali, R. et al. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Mod Pathol 27, 502–508 (2014). https://doi.org/10.1038/modpathol.2013.168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.168
Keywords
This article is cited by
-
Systematic Review and Case Series of Pleomorphic Dermal Sarcoma: an Uncommon Cutaneous Pathology Predominantly in the Head and Neck
SN Comprehensive Clinical Medicine (2022)
-
TERT promoter mutation in sebaceous neoplasms
Virchows Archiv (2021)
-
Soft Tissue Special Issue: Cutaneous Pleomorphic Spindle Cell Tumors
Head and Neck Pathology (2020)
-
The Angiosarcoma Project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research
Nature Medicine (2020)
-
Genome-wide methylation profiling and copy number analysis in atypical fibroxanthomas and pleomorphic dermal sarcomas indicate a similar molecular phenotype
Clinical Sarcoma Research (2019)