Abstract
Tumor ‘budding’, loosely defined by the presence of individual cells and small clusters of tumor cells at the invasive front of carcinomas, has received much recent attention, particularly in the setting of colorectal carcinoma. It has been postulated to represent an epithelial–mesenchymal transition. Tumor budding is a well-established independent adverse prognostic factor in colorectal carcinoma that may allow for stratification of patients into risk categories more meaningful than those defined by TNM staging, and also potentially guide treatment decisions, especially in T1 and T3 N0 (Stage II, Dukes’ B) colorectal carcinoma. Unfortunately, its universal acceptance as a reportable factor has been held back by a lack of definitional uniformity with respect to both qualitative and quantitative aspects of tumor budding. The purpose of this review is fourfold: (1) to describe the morphology of tumor budding and its relationship to other potentially important features of the invasive front; (2) to summarize current knowledge regarding the prognostic significance and potential clinical implications of this histomorphological feature; (3) to highlight the challenges posed by a lack of data to allow standardization with respect to the qualitative and quantitative criteria used to define budding; and (4) to present a practical approach to the assessment of tumor budding in everyday practice.
Similar content being viewed by others
Main
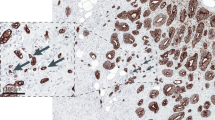
Colorectal carcinoma is one of the commonest human cancers, and one of the leading causes of cancer-related death.1 Prognosis and treatment decisions are based primarily on the extent of the disease, as codified by the TNM staging system. Unfortunately, a substantial number of tumors behave poorly despite being categorized as low risk based on their TNM stage.2 Thus, the search for additional prognostic factors in the assessment of colorectal carcinoma has been a major research focus. Of the histopathological factors studied to date, the most promising include extramural venous invasion, the nature of the advancing front (pushing vs infiltrative), an inflammatory infiltrate, microsatellite instability, and tumor budding—the presence of small discrete clusters of tumor cells at the invasive edge (Figure 1). There is now overwhelming evidence that tumor budding is an independent prognostic factor in colorectal carcinoma, particularly in node-negative disease. Thus, its assessment has the potential to enhance prognostic accuracy and influence treatment algorithms. When examined carefully, the majority of colorectal carcinomas display some degree of budding; hence, attempts have been made at developing scoring systems to identify a prognostically significant degree of budding, commonly termed ‘high-grade’ budding. Definitions of high-grade budding vary substantially among different authors and even among different studies by the same authors.
Tumor budding in colorectal carcinoma (a) Illustration of a colorectal carcinoma with an infiltrative border but no budding. Tumor buds, defined as individual cells or small clusters (<5 cells) of tumor cells at the invasive front (arrows), are illustrated in these examples from (b) a malignant polyp and (c and d) a colorectal cancer resection specimen. Note the blurring of the tumor–stroma interface (c), which corresponds with tumor budding at higher magnification (d). (Original magnification: a— × 100; b— × 200; c— × 20; d— × 400).
Although tumor budding only entered the mainstream pathology literature in the past decade, it was first described in the 1950s by Imai,3 who postulated that the presence of ‘sprouting’ at the invasive edge of carcinomas reflected a more rapid tumor growth rate (or ‘Schub’ phase of growth). Budding has been described in association with carcinomas at a number of different sites, but it has been most extensively studied in colorectal carcinoma.
The biology of tumor budding is not understood but the prevailing theory is that at least some types of budding represent an example of epithelial–mesenchymal transition, a process thought to occur physiologically during embryological development, and pathologically during fibrosis and tumor invasion.4, 5 The epithelial–mesenchymal transition is characterized by loss of cell adhesion molecules, cytoskeletal alterations, increased production of extracellular matrix components, resistance to apoptosis, and ability to degrade basement membrane, resulting in a phenotype with increased migratory capacity and invasiveness.6, 7 Current theories relating to the epithelial–mesenchymal transition and tumor budding have been comprehensively reviewed elsewhere, most recently by Zlobec et al,8 and will not be discussed here.
The purpose of this review is to examine practical issues related to tumor budding that may be of interest to the practicing pathologist. We will discuss the morphological features and prognostic significance of tumor budding and summarize the various scoring systems for its assessment in order to highlight problems related to definitional heterogeneity in tumor-budding studies.
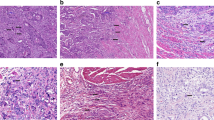
Finally, we will present a practical approach to the assessment of tumor budding. Although performing a tumor bud count is easy in theory, deciding what is or is not a bud can prove surprisingly difficult in practice. Buds are often obscured in the setting of a prominent inflammatory reaction; fibroblasts and stromal cells may occasionally be mistaken for buds; and single-cell buds are usually inconspicuous and often missed on H&E evaluation (Figure 2).
‘Challenging scenarios’. (a) Peritumoral inflammatory cells, including histiocytes, can be difficult to differentiate from tumor buds, and may sometimes obscure the underlying budding. (b) Immunohistochemistry for anti-cytokeratin may help to highlight tumor buds in this setting. (c) Given that tumor budding may represent an epithelial–mesenchymal transformation, their separation from stromal cells can also be challenging and (d) anti-cytokeratin stains may be very useful in identifying tumor buds. (e) Fragmentation of neoplastic glandular structures (arrow) can sometimes give the impression of extensive tumor budding on H&E and on immunohistochemistry with (f) anti-cytokeratin. In this example, the small fragments/individual tumor cells highlighted by anti-cytokeratin in the region of the fragmented gland (arrow between fragments) may be a consequence of fragmentation rather than true budding. (Original magnification: a— × 40; b— × 40; c— × 100; d— × 100; e— × 200; f— × 200).
Morphological features
Although tumor budding (‘sprouting’) was originally described by Imai in the 1950s, the first detailed description of tumor buds in the English language literature was put forth by Gabbert et al9 who used light and electron microscopy to characterize what they termed ‘tumor dedifferentiation’ at the invasive edge of colorectal carcinomas. They identified a subset of colorectal carcinomas with invasive fronts that displayed a strikingly different architecture compared with the central area of the tumor, with well-developed glandular structures giving way to isolated tumor cells and small cell groups, as illustrated in Figure 1. At the ultrastructural level, these small clusters and isolated tumor cells appeared poorly differentiated, with large nuclei and a loss of microvilli and basement membrane, and poorly developed or absent junctional complexes and desmosomes.
When seen in a single section, tumor buds appear as clusters of cells that have broken off from the main tumor mass. By examining serial sections of high budding tumors stained with anti-cytokeratin antibodies, Prall et al10 demonstrated that most buds that appear to represent discrete clusters of cells are in fact connected to adjacent larger glands. Indeed, Morodomi et al11 coined the term ‘budding’ because the undifferentiated single cells and tubular tumor nests that they counted as buds both appeared to be budding from larger neoplastic glands.
Shinto et al12, 13 used immunohistochemistry with anti-cytokeratin antibodies to highlight another feature of buds, termed cytoplasmic pseudofragmentation, whereby cytoplasmic fragments, visible only by immunohistochemistry, are seen in the immediate vicinity of tumor buds. When examined on serial sections, some of the fragments were shown to be connected to the buds (hence the term ‘pseudofragments’). The presence of pseudofragments is associated with high-grade budding, but was shown to be an independent prognostic factor on multivariate analysis, suggesting that its presence signifies an aggressive budding phenotype.
Although tumor buds are usually most prominent at the invasive front, intratumoral budding has been described as well. Only one study has attempted to assess the significance of this finding: Lugli et al14 reported that the presence of intratumoral budding was strongly correlated with peritumoral budding, but was found to be an independent prognostic factor on multivariate analysis.
There is a strong association between budding and the presence of lymph node metastases and lymphovascular invasion,11, 12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 defined by the presence of tumor cells within an endothelium-lined space, and it has been suggested that buds represent the part of the tumor that has gained the ability to invade lymphatics and vascular channels. This idea is supported by two intriguing morphological studies: Morodomi et al33 examined serial sections of high-budding areas to demonstrate that budding nests are often found adjacent to areas of lymphovascular space invasion, and, in a more recent study, Ohtsuki et al31 performed double staining for anti-cytokeratin antibodies and anti-lymphatic antibodies, finding that a number of ‘buds’ at the invasive edge of a tumor are in fact located in small lymphatic spaces. Similarly, the presence of budding has been associated with increased risk of distant metastases,34, 35, 36 suggesting that budding may also be associated with vascular invasion. A few tumor-budding studies have used vascular markers and/or elastic stains to assess vascular invasion,11, 12, 19, 22, 26, 32, 37, 38 but only four have analyzed the relationship between budding and vascular invasion: Kazama et al22 found no relationship between budding and vascular invasion, whereas three other studies have reported a statistically significant correlation between budding and venous invasion, though the association was not as pronounced as the relationship with lymphatic invasion and lymph node metastases.11, 12, 32
The tumor–host interaction at the invasive front may be of prognostic importance in the setting of tumor budding. Several studies have shown peritumoral lymphocytic infiltration to be an independent prognostic factor in colorectal carcinoma. Lugli et al23 have demonstrated that a peritumoral lymphocytic reaction is associated with improved prognosis in the setting of tumor budding, suggesting that the immune response might target the tumor buds. The nature of the stroma at the invasive margin may provide further prognostic information, with myxoid stroma being associated with worse prognosis,20, 39 but further studies are needed to confirm the reproducibility and prognostic importance of these findings.
Clinical significance of tumor budding
Tumor budding is associated with other histopathological factors known to portend a worse prognosis, namely higher tumor grade, infiltrating tumor border, the presence of lymphovascular and perineural invasion, and lymph node metastases.11, 12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 On multivariate analysis, budding has consistently emerged as an independent adverse prognostic factor, associated with local tumor recurrence and distant metastases, and significantly worse overall and disease-free survival.12, 15, 16, 21, 27, 28, 31, 32, 34, 35, 36, 38, 40, 41
The adverse prognostic impact of high-grade tumor budding is seen in both early and advanced colorectal carcinoma, and there are several scenarios in which this feature might influence clinical decision making, particularly in early colorectal carcinoma.
Tumor Budding in Early Colorectal Carcinoma
Submucosally invasive colorectal carcinoma is associated with excellent outcomes and low rates of lymph node metastases, and a subset of patients can be successfully managed with endoscopic mucosal resection or polypectomy,42 thus avoiding the risks associated with segmental resection. Identification of candidates for endoscopic resection rests mainly on the absence of certain high-risk histopathological features, namely high tumor grade, lymphovascular invasion, and tumor budding.26, 42 Tumor extending to the cauterized margin can often be managed by endoscopic follow up.
Several large studies have shown tumor budding in submucosally invasive colorectal carcinoma to be an independent prognostic factor associated with lymph node metastases, local recurrence, and cancer-related death.16, 19, 22, 25, 26, 29, 30, 37, 43, 44 In the largest of these studies, Ueno et al26 demonstrated that, in submucosally invasive colorectal carcinoma, high tumor grade, lymphovascular invasion, and tumor budding are the three factors independently associated with lymph node metastases. Patients without any of these three features showed exceptionally low rates of lymph node metastases (1%, 1/138); in the presence of one risk factor, the rate of nodal metastases increased substantially to 21% (12/58), and when two or three factors were present, the risk was 36% (20/55), suggesting that in the absence of these factors, polypectomy alone is sufficient treatment for early colorectal carcinoma provided the resection margins are clear. In this study, Ueno et al further showed that in the absence of extensive submucosal invasion (defined as ≤4 mm wide and ≤2 mm deep), the risk of nodal metastases (including isolated tumor cells, identifiable only with anti-cytokeratin antibodies) in tumors lacking the three above-mentioned features was 0; thus, extent of submucosal invasion may need to be included in the histopathological assessment of T1 tumors if strict criteria are to be applied for the identification of patients that can safely be managed without surgical resection.
Tumor Budding in Stage II (T3–4 N0) Colorectal Carcinoma
As patients with Stage II colorectal carcinoma have highly variable outcomes, tumor budding may be particularly useful in identifying high-risk subgroups within this population. In one of the earlier studies of tumor budding, Hase et al18 demonstrated that 5-year survival rates of Dukes B (stage II, T3–4 N0) patients with high-grade budding are significantly worse than those of Dukes C (N+) patients without budding (29 percent vs 66 percent; P<0.001). A number of more recent studies have confirmed that patients with Stage II colorectal carcinoma do significantly worse when high-grade budding is present,34, 35, 36, 38, 41, 45 and several studies have shown that survival rates of patients with Stage II colorectal carcinoma with high-grade budding are equivalent to survival rates of patients with Stage III colorectal carcinoma.34, 35, 36 In their studies of Stage II and III pT3 tumors, Okuyama et al34, 35 found that tumor budding was the only factor on multivariate analysis to be associated with decreased survival and was more prognostically significant than lymph node metastases.
Currently, the indications for chemotherapy in Stage II colorectal carcinoma are unclear, and adjuvant therapy is considered only in subgroups with adverse prognostic features, eg, high-histological grade, pT4 disease, and extramural venous invasion. The QUASAR trial demonstrated that chemotherapy does result in increased survival in Stage II patients, but the improvement was small and may not justify the costs and side effects of chemotherapy in all patients.46 The significantly worse outcome experienced by Stage II patients with tumor budding has prompted some authors to suggest that adjuvant chemotherapy should be considered in these patients,18, 34, 35, 36, 41 but there are no published data assessing the effectiveness of chemotherapy in Stage II colorectal carcinoma with tumor budding.
Tumor Budding in Stage III (T1–4 N+) Colorectal Carcinoma
Data regarding budding in Stage III colorectal carcinoma are limited, and the two studies reporting on the prognostic significance of budding in this subgroup of patients have produced conflicting results. Choi et al15 studied tumor budding in 103 patients with Stage III rectal carcinoma; budding was an independent poor prognostic factor: when added to N stage as a prognostic variable, there was better prognostic stratification with respect to 5-year disease-free survival compared with the Americal Joint Comittee on Cancer nodal staging alone. On the other hand, in a series of 477 Stage III colorectal carcinomas reported on by Sy et al,47 although there was a survival difference between high- and low-budding groups, on multivariate analysis budding was not found to be an independent prognostic factor. Although more studies are needed to define the prognostic significance of tumor budding in this group, it is not likely that the reporting of this factor will impact the clinical management of these patients.
Tumor Budding in Metastatic Colorectal Carcinoma
The significance of tumor budding in metastatic colorectal carcinoma has received little attention. However, one study showed that the presence of tumor budding predicts poor response to anti-EGFR therapy in patients with metastatic colorectal carcinoma: Zlobec et al48 studied a series of 43 patients with metastatic colorectal carcinoma treated with cetuximab or penitumumab, and found all patients with a KRAS mutation (n=7) and/or high-grade tumor budding (n=11, including 4 with KRAS mutation) to be nonresponsive to anti-EGFR therapy. Although this finding needs to be confirmed in larger cohorts, it raises the interesting possibility that tumor budding in addition to K-RAS analysis may more accurately determine eligibility for anti-EGFR therapy.
Tumor Budding in Microsatellite Unstable Tumors
The association between tumor budding and poor prognosis in sporadic microsatellite stable colorectal carcinoma is well established, but whether this can be extrapolated to sporadic and Lynch syndrome-related high frequency microsatellite unstable colorectal carcinoma is unclear. Many studies of tumor budding have excluded Lynch syndrome-related cases, while others have not attempted to analyze the groups separately. Only a few studies have compared the frequency of tumor budding in these different types of colorectal carcinomas: in these studies, the frequency of tumor budding was approximately 50% in sporadic microsatellite stable and low-frequency microsatellite unstable tumors; lower rates of tumor budding were seen in Lynch syndrome-associated tumors (∼20%); whereas tumor budding was found to be virtually absent in sporadic high-frequency microsatellite unstable colorectal carcinoma.23, 49, 50 It has been postulated that the relative infrequency of budding in microsatellite unstable tumors may, at least in part, explain the relatively better prognosis of these tumors.49 Interestingly, compared with mismatch repair-proficient tumors, mismatch repair-deficient tumors have been shown to display less cytoplasmic pseudofragmentation in the context of high-grade budding,51 and to be associated with less intratumoral budding,14 both of which are associated with a worse prognosis. Given the differing biological pathways of microsatellite stable, sporadic high-frequency microsatellite unstable and Lynch syndrome-related tumors, it seems likely that the frequency and clinical significance of tumor budding will vary significantly among these three groups, but awaits confirmation in controlled studies.
Scoring systems
Although a large number of studies have shown tumor budding to be an independent adverse prognostic factor in colorectal carcinoma, study methodologies have varied widely. Different authors have used different scoring systems as well as differing definitions as to what constitutes a bud. The most widely cited scoring system, developed by Ueno et al,27 defines ‘high-grade budding’ as >10 buds composed of fewer than 5 cells in a 25 × field (field area=0.385 mm2). Other authors have defined buds as being composed of ≤5 cells,38 while some have not set an upper cell limit and instead count both single cells and bundles of five or more cells as buds, as proposed by Morodomi et al11 and commonly applied in the Japanese literature. This heterogeneity in defining budding is compounded by the use of varying field diameters and cutoffs for defining ‘high-grade’ budding, especially when a score is given as #/hpf rather than/mm2. Although most authors have used Ueno's definition of ‘high-grade budding’ (>10 buds of fewer than 5 cells), all have used a 20 × objective, rather than the 25 × objective used by Ueno et al, resulting in significantly greater field areas than that originally reported by Ueno. Furthermore, while the majority of studies have evaluated only areas with the greatest amount of budding, others have proposed calculating an average bud count,11 counting the number of buds along the entire invasive front19, 24, 52, 53 or calculating the proportion of invasive front with tumor budding.21, 36, 40 Such methodological heterogeneity is illustrated in Table 1, which summarizes the bud counting methods used in a sample of tumor-budding studies.
Establishing a Prognostically Significant Cutoff for ‘High-Grade’ Budding
Although Ueno's definition of ‘high-grade’ budding (ie, 10 buds in a 25 × field) is the most widely applied in the literature, there is no evidence that this is the optimal cutoff for evaluating budding. Only a handful of studies have attempted to analyze their data so as to generate a clinically useful threshold for the assessment of high-grade budding. The results and methodologies of these studies are summarized in Table 2. In addition, Masaki et al24, 53 have proposed a formula based on the total number of buds counted along the entire invasive margin to predict the probability of lymph node metastases in T1 and T2 colorectal carcinoma: although this is an interesting idea, it is not practical for use in everyday practice.
Subjectivity in the Qualitative Assessment of Tumour Budding
In addition to the quantitative issues of inconsistent cutoffs and differing field diameters, there are a number of qualitative considerations contributing to subjectivity in defining a tumour bud. Although only a few authors have specifically commented on this problem, the identification of tumor buds can prove very difficult in several circumstances, eg, (1) stromal cells or histiocytes masquerading as buds, (2) marked inflammation obscuring buds, (3) difficulties in determining whether a small cluster of cells represents a true bud or mechanical fragmentation of a larger gland, etc (Figure 2). Most studies of tumor budding have not provided much detail regarding qualitative criteria used to include or exclude a potential ‘bud’. In addition, there appear to be discrepancies in the qualitative assessment of budding, even among studies ostensibly using the same objective criteria of clusters of <5 cells: eg, while Jass et al49 counted only ‘discrete clusters’ of cells as buds, Ha et al17 defined buds as clusters ‘appearing to bud from a larger gland’, suggesting that they may have also counted buds that were not completely separate from adjacent glands. Although such variability may be reduced to some degree by establishing consensus criteria, there will likely remain a subset of cases in which budding will be difficult to evaluate owing to the resemblance of buds to surrounding stromal cells and/or the concealment of buds in the setting of a prominent peritumoral inflammatory reaction (Figure 2).
Role of Immunohistochemistry in the Evaluation of Budding
Some authors have attempted to eliminate the subjectivity inherent in bud counting by using immunohistochemistry with anti-cytokeratin antibodies to highlight buds and distinguish them from surrounding stromal cells.12, 13, 22, 23, 31, 32, 38, 40, 43, 44, 48, 51, 54, 55 The use of immunohistochemistry in the assessment of budding is somewhat controversial: some authors argue that the evaluation of budding should be limited to H&E-stained slides because of the cost and impracticality of performing immunohistochemistry in routine cases, while others argue that immunohistochemistry should be used routinely to improve the accuracy and reproducibility of bud counts. In addition to cost considerations, the use of immunohistochemistry may also require the establishment of a separate cutoff for defining positive budding. One study comparing the bud counts obtained on H&E to those obtained with immunohistochemistry, not surprisingly, found significantly higher counts with the latter.31 Indeed, the few studies that have attempted to define an optimal budding threshold on anti-cytokeratin-stained slides have reported higher cutoffs than those found to be prognostically significant on H&E (summarized in Table 2).
Currently, the role for immunohistochemistry in the evaluation of tumor budding is unclear, and further studies are needed to assess the relationship between bud counts obtained on H&E vs those obtained with anti-cytokeratin antibodies, and to determine the most prognostically useful cutoff for bud counting with both H&E and anti-cytokeratin antibodies.
Interobserver Variability in the Assessment of Tumor Budding
In light of these myriad sources of subjectivity, one would expect the evaluation of budding to suffer from suboptimal interobserver variability. A few studies on tumor budding have incorporated an assessment of interobserver variability, with reported kappa values ranging from 0.41 to 0.938.15, 27, 32, 34, 38, 41, 48 Kappa values, a commonly used measure of interobserver agreement, can be interpreted as follows: <0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and >0.80, very good.56
Intuitively, one would expect the use of immunohistochemistry to improve interobserver variability, but studies assessing interobserver variability on immunohistochemically stained slides have reported kappa values ranging from 0.53 to 0.874,32, 38, 48 similar to those reported by authors assessing tumour budding on H&E.15, 27, 32, 34, 41 Only one study has directly compared interobserver variability between assessments performed on H&E and immunohistochemically stained slides among the same group of pathologists: when Suzuki et al32 evaluated budding with both H&E and immunohistochemistry, they showed only a modest improvement in interobserver variability when anti-cytokeratin antibodies were used (κ=0.41 with H&E, and κ=0.53 with immunohistochemistry).
Practical considerations in the assessment and reporting of tumor budding
Opinions are divided as to whether tumor budding should be recorded in the pathological assessment of colorectal carcinoma. Although the College of American Pathologists has not included budding in its checklist of reportable features, both the Association of Directors of Anatomic and Surgical Pathology and the Union for International Cancer Control recommend its inclusion in routine reporting.57, 58
Our institutional policy is to report the presence or absence of tumor budding in all malignant polyps and colorectal cancer resection specimens. Although we recognize the limitations posed by a lack of definitional uniformity, we feel that its assessment is important both for the purposes of data gathering, and, in the setting of malignant polyps, for guiding treatment decisions.
Until universally accepted criteria are established, we apply those of Ueno et al which are not only of proven prognostic significance, but are also easily applied and are the most widely used criteria in the budding literature. We report tumor budding as present if ≥10 groups of <5 cells are counted in a 20 × objective field (ie, Ueno's so-called ‘high-grade budding’).27 In borderline cases, where only 5–10 definite buds/20 × field are identified but there are additional possible tumor cells that cannot be confidently differentiated from stroma or histiocytes, a cytokeratin stain is performed. If this confirms the impression of additional tumor cells, bringing the count to ≥10 buds, we report as positive for tumor budding. However, we caution against the routine use of cytokeratin stains in cases where bud counts on H&E do not approach 10/20 × objective. In our experience, counts by cytokeratin immunohistochemistry are substantially higher than those on H&E and the limited data suggest that much higher cutoffs are needed to reach prognostic significance.23, 38, 48 As tumor budding is most prominent at the invasive tumor front, we concentrate on, but do not restrict our assessment to, this area.
At scanning power, clues to the presence of tumor budding include:
-
a)
infiltrating growth pattern;
-
b)
marked irregularity at invasive front; and
-
c)
blurring of the interface between tumor and underlying stroma.
The presence of these features prompts close evaluation at medium and high magnification.
In our experience, there are several scenarios in which tumor-budding assessment is challenging:
-
a)
abundance of histiocytes and activated lymphocytes at invasive front (Figure 2a);
-
b)
prominent stromal reaction (Figure 2c);
-
c)
glandular fragmentation associated with a marked acute inflammatory infiltrate;
-
d)
tumour fragments floating in mucin pools; and
-
e)
fragmented glands with surrounding retraction artifact (Figure 2e)
In scenarios (a) and (b), a cytokeratin stain is used to distinguish between tumor cells and stromal/inflammatory cells (Figures 2b and d). In scenarios (c) to (e), the fragmented cells are excluded from bud counts.
We believe it is particularly important to report tumour budding in the setting of malignant polyps, as this finding increases the risk of nodal metastasis to around 15–20%,26 and should prompt consideration of a segmental resection with lymphadenectomy. As such, we make a point of commenting not only on its presence, but on its associated risk of nodal metastasis (as we do for lymphovascular invasion and a poorly differentiated component).
Our approach will undoubtedly need to be refined once optimal evidence-based guidelines are established. However, based on the available evidence, and in an area lacking standardization, this strategy provides institutional guidelines and consistency for reporting of tumour budding.
Conclusions and future directions
There is strong evidence to suggest that tumor budding is an adverse prognostic factor that can help to stratify patients into more meaningful risk groups than TNM staging alone, and, even more importantly, has the potential to guide decision making. Its presence in T1 colorectal carcinoma, when evaluated in conjunction with other prognostically significant clinical and histopathological features, can identify a subset of high-risk patients requiring segmental resection including nodes. In the setting of Stage II colorectal carcinoma, the presence of budding has been associated with significantly worse outcomes, and if this subset of patients is shown to derive a survival benefit from chemotherapy, the evaluation of budding could potentially assume an important role in treatment algorithms in Stage II colorectal carcinoma.
In order for the considerable prognostic power of tumor budding to be fully realized, consensus criteria for its evaluation must be established, both to guide further research in this area and to provide the practicing pathologist with guidelines for its reporting. With respect to setting these criteria, studies focusing on budding should be designed to determine, if possible, the optimal quantitative cutoff for defining clinically significant tumor budding. Tumor budding is a continuous variable; thus, any cutoff is bound to be at least somewhat arbitrary, but an attempt must be made at defining the budding threshold that will provide the most clinically relevant prognostic and predictive information. Furthermore, authors reporting on tumor budding should provide more detailed information regarding the qualitative and quantitative criteria used to evaluate budding in order to allow for meaningful comparisons among different studies. Finally, the role of immunohistochemistry in the evaluation of budding needs to be clarified. Although it is probably unnecessary and impractical to perform immunohistochemistry on all colorectal carcinoma cases, there may be certain situations, particularly in the context of a prominent peritumoral inflammatory reaction, where a cytokeratin stain may reveal buds that are obscured on H&E. Criteria for immunohistochemical evaluation therefore also need to be established.
The greatest obstacle to the adoption of budding as a routinely reportable feature is the lack of well-defined, evidence-based criteria for its assessment. Given the overwhelming evidence that tumor budding is one of the most powerful prognostic factors at our disposal, it is incumbent on the GI pathology community to move swiftly to address and remove the barriers to its universal reporting.
References
Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics, 2001. CA Cancer J Clin 2001;51:15–36.
Puppa G, Sonzogni A, Colombari R, et al. TNM staging system of colorectal carcinoma: a critical appraisal of challenging issues. Arch Pathol Lab Med 2010;134:837–852.
Imai T . The growth of human carcinoma: a morphological analysis. Fukuoka Igaku Zasshi 1954;45:30.
Chaffer CL, Weinberg RA . A perspective on cancer cell metastasis. Science 2011;331:1559–1564.
Kalluri R . EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 2009;119:1417–1419.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420–1428.
Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009;119:1438–1449.
Zlobec I, Lugli A . Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 2010;1:651–661.
Gabbert H, Wagner R, Moll R, et al. Tumor dedifferentiation: an important step in tumor invasion. Clin Exp Metastasis 1985;3:257–279.
Prall F, Ostwald C, Linnebacher M . Tubular invasion and the morphogenesis of tumor budding in colorectal carcinoma. Hum Pathol 2009;40:1510–1512.
Morodomi T, Isomoto H, Shirouzu K, et al. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer 1989;63:539–543.
Shinto E, Jass JR, Tsuda H, et al. Differential prognostic significance of morphologic invasive markers in colorectal cancer: tumor budding and cytoplasmic podia. Dis Colon Rectum 2006;49:1422–1430.
Shinto E, Mochizuki H, Ueno H, et al. A novel classification of tumour budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology 2005;47:25–31.
Lugli A, Vlajnic T, Giger O, et al. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum Pathol 2011;42:1833–1840.
Choi HJ, Park KJ, Shin JS, et al. Tumor budding as a prognostic marker in stage-III rectal carcinoma. Int J Colorectal Dis 2007;22:863–868.
Guzinska-Ustymowicz K . The role of tumour budding at the front of invasion and recurrence of rectal carcinoma. Anticancer Res 2005;25:1269–1272.
Ha SS, Choi HJ, Park KJ, et al. Intensity of tumor budding as an index for the malignant potential in invasive rectal carcinoma. Cancer Res Treat 2005;37:177–182.
Hase K, Shatney C, Johnson D, et al. Prognostic value of tumor ‘budding’ in patients with colorectal cancer. Dis Colon Rectum 1993;36:627–635.
Homma Y, Hamano T, Otsuki Y, et al. Severe tumor budding is a risk factor for lateral lymph node metastasis in early rectal cancers. J Surg Oncol 2010;102:230–234.
Kajiwara Y, Ueno H, Hashiguchi Y, et al. Risk factors of nodal involvement in T2 colorectal cancer. Dis Colon Rectum 2010;53:1393–1399.
Kanazawa H, Mitomi H, Nishiyama Y, et al. Tumour budding at invasive margins and outcome in colorectal cancer. Colorectal Dis 2008;10:41–47.
Kazama S, Watanabe T, Ajioka Y, et al. Tumour budding at the deepest invasive margin correlates with lymph node metastasis in submucosal colorectal cancer detected by anticytokeratin antibody CAM5.2. Br J Cancer 2006;94:293–298.
Lugli A, Karamitopoulou E, Panayiotides I, et al. CD8+ lymphocytes/tumour-budding index: an independent prognostic factor representing a ‘pro-/anti-tumour’ approach to tumour host interaction in colorectal cancer. Br J Cancer 2009;101:1382–1392.
Masaki T, Matsuoka H, Sugiyama M, et al. Tumor budding and evidence-based treatment of T2 rectal carcinomas. J Surg Oncol 2005;92:59–63.
Sohn DK, Chang HJ, Park JW, et al. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol 2007;60:912–915.
Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004;127:385–394.
Ueno H, Murphy J, Jass JR, et al. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002;40:127–132.
Ueno H, Price AB, Wilkinson KH, et al. A new prognostic staging system for rectal cancer. Ann Surg 2004;240:832–839.
Yamauchi H, Togashi K, Kawamura YJ, et al. Pathological predictors for lymph node metastasis in T1 colorectal cancer. Surg Today 2008;38:905–910.
Wang HS, Liang WY, Lin TC, et al. Curative resection of T1 colorectal carcinoma: risk of lymph node metastasis and long-term prognosis. Dis Colon Rectum 2005;48:1182–1192.
Ohtsuki K, Koyama F, Tamura T, et al. Prognostic value of immunohistochemical analysis of tumor budding in colorectal carcinoma. Anticancer Res 2008;28:1831–1836.
Suzuki A, Togashi K, Nokubi M, et al. Evaluation of venous invasion by Elastica van Gieson stain and tumor budding predicts local and distant metastases in patients with T1 stage colorectal cancer. Am J Surg Pathol 2009;33:1601–1607.
Morodomi T . Clinicopathological studies of advanced rectal cancers—prediction of the degree of lymph node metastasis from histopathological finding of pre-operative biopsy specimens. Nippon Geka Gakkai Zasshi 1988;89:352–364.
Okuyama T, Oya M, Ishikawa H . Budding as a useful prognostic marker in pT3 well- or moderately-differentiated rectal adenocarcinoma. J Surg Oncol 2003;83:42–47.
Okuyama T, Nakamura T, Yamaguchi M . Budding is useful to select high-risk patients in stage II well-differentiated or moderately differentiated colon adenocarcinoma. Dis Colon Rectum 2003;46:1400–1406.
Nakamura T, Mitomi H, Kanazawa H, et al. Tumor budding as an index to identify high-risk patients with stage II colon cancer. Dis Colon Rectum 2008;51:568–572.
Yasuda K, Inomata M, Shiromizu A, et al. Risk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resection. Dis Colon Rectum 2007;50:1370–1376.
Prall F, Nizze H, Barten M . Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology 2005;47:17–24.
Ueno H, Jones AM, Wilkinson KH, et al. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut 2004;53:581–586.
Syk E, Lenander C, Nilsson PJ, et al. Tumour budding correlates with local recurrence of rectal cancer. Colorectal Dis 2011;13:255–262.
Wang LM, Kevans D, Mulcahy H, et al. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 2009;33:134–141.
Tytherleigh MG, Warren BF, Mortensen NJ . Management of early rectal cancer. Br J Surg 2008;95:409–423.
Ishikawa Y, Akishima-Fukasawa Y, Ito K, et al. Histopathologic determinants of regional lymph node metastasis in early colorectal cancer. Cancer 2008;112:924–933.
Losi L, Ponti G, Gregorio CD, et al. Prognostic significance of histological features and biological parameters in stage I (pT1 and pT2) colorectal adenocarcinoma. Pathol Res Pract 2006;202:663–670.
Tanaka M, Hashiguchi Y, Ueno H, et al. Tumor budding at the invasive margin can predict patients at high risk of recurrence after curative surgery for stage II, T3 colon cancer. Dis Colon Rectum 2003;46:1054–1059.
Gray R, Barnwell J, McConkey C, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–2029.
Sy J, Fung CL, Dent OF, et al. Tumor budding and survival after potentially curative resection of node-positive colon cancer. Dis Colon Rectum 2010;53:301–307.
Zlobec I, Molinari F, Martin V, et al. Tumor budding predicts response to anti-EGFR therapies in metastatic colorectal cancer patients. World J Gastroenterol 2010;16:4823–4831.
Jass JR, Barker M, Fraser L, et al. APC mutation and tumour budding in colorectal cancer. J Clin Pathol 2003;56:69–73.
Kevans D, Wang LM, Sheahan K, et al. Epithelial-Mesenchymal Transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int J Surg Pathol 2011;19:751–760.
Shinto E, Baker K, Tsuda H, et al. Tumor buds show reduced expression of laminin-5 gamma 2 chain in DNA mismatch repair deficient colorectal cancer. Dis Colon Rectum 2006;49:1193–1202.
Mikami T, Yoshida T, Numata Y, et al. Invasive behavior of ulcerative colitis-associated carcinoma is related to reduced expression of CD44 extracellular domain: comparison with sporadic colon carcinoma. Diagn Pathol 2011;6:30.
Masaki T, Matsuoka H, Sugiyama M, et al. Actual number of tumor budding as a new tool for the individualization of treatment of T1 colorectal carcinomas. J Gastroenterol Hepatol 2006;21:1115–1121.
Ogawa T, Yoshida T, Tsuruta T, et al. Tumor budding is predictive of lymphatic involvement and lymph node metastases in submucosal invasive colorectal adenocarcinomas and in non-polypoid compared with polypoid growths. Scand J Gastroenterol 2009;44:605–614.
Prall F, Ostwald C . High-degree tumor budding and podia-formation in sporadic colorectal carcinomas with K-ras gene mutations. Hum Pathol 2007;38:1696–1702.
Altman DG . Practical Statistics for Medical Research, Vol, Chapman and Hall: London, 1991.
Turner RR, Li C, Compton CC . Newer pathologic assessment techniques for colorectal carcinoma. Clin Cancer Res 2007;13:6871s–6876s.
Jass JR, O’Brien MJ, Riddell RH, et al. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma. Virchows Arch 2007;450:1–13.
Acknowledgements
We thank Dr Masanori Tanaka (Department of Pathology, City Hospital, Hirosaki, Japan) for assistance in obtaining and translating Japanese articles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mitrovic, B., Schaeffer, D., Riddell, R. et al. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol 25, 1315–1325 (2012). https://doi.org/10.1038/modpathol.2012.94
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.94
Keywords
This article is cited by
-
Tumor budding as an indicator for lymph node metastasis and prognosis of early gastric cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Is there no need to discuss adjuvant chemotherapy in stage II colon cancer patients with high tumor budding and lymphovascular invasion?
Langenbeck's Archives of Surgery (2023)
-
Composite scoring system and optimal tumor budding cut-off number for estimating lymph node metastasis in submucosal colorectal cancer
BMC Cancer (2022)
-
The peri- and intratumoral immune cell infiltrate and PD-L1 status in invasive squamous cell carcinomas of the penis
Clinical and Translational Oncology (2022)
-
Continuous formation of small clusters with LGR5-positive cells contributes to tumor growth in a colorectal cancer xenograft model
Laboratory Investigation (2021)