Abstract
This study assessed whether analysis of MDM2 copy number by fluorescence in situ hybridization (FISH) would help distinguish lipomas from atypical lipomatous tumors, otherwise referred to as well-differentiated liposarcomas, using a commercially available MDM2 FISH kit. 227 lipomatous and 201 non-lipomatous tumors were analyzed to assess its sensitivity and specificity. Of 178 mature lipomatous tumors, 86 were classified histologically as lipoma and 92 as atypical lipomatous tumor. Two of the lipomas harboring MDM2 amplification were reclassified as atypical lipomatous tumors. Overall, 13 atypical lipomatous tumors did not reveal MDM2 or CDK4 amplification, although this was reduced to 12 following analysis of multiple slides. Three of these cases revealed very occasional tumor cells harboring high-level MDM2 amplification, two had a dedifferentiated component, and MDM2 amplification was detected when one tumor recurred. The remaining six cases exhibited reactive/inflammatory features and were reclassified as lipomas. The findings indicate that MDM2 amplification is 93.5% sensitive for diagnosing atypical lipomatous tumor. A total of 2 of the 20 dedifferentiated liposarcomas failed to reveal MDM2 amplification. All atypical lipomatous tumors measured >10 cm, two dedifferentiated liposarcoma presented de novo at <10 cm, and ∼50% of lipomas measured >10 cm. Spindle cell lipomas, lipoblastomas, hibernomas and pleomorphic liposarcomas did not reveal MDM2 amplification. Of 201 non-lipomatous tumors, eight revealed MDM2 amplification or multiple faint alphoid 12 signals and were reclassified as dedifferentiated liposarcoma. Multiple faint alphoid 12 signals were observed in nine tumors from seven patients, an observation not previously reported on paraffin sections: these included four atypical lipomatous tumors, and three dedifferentiated liposarcomas, one previously diagnosed as a myxofibrosarcoma, all of which also revealed amplification of CDK4, although two lacked MDM2 amplification. MDM2 FISH test is a useful adjunct to histology for distinguishing lipoma from atypical lipomatous tumor. The limitations of molecular genetic tests must be known before introducing them into a clinical service.
Similar content being viewed by others
Main
The most common lipomatous neoplasms are the well-differentiated group including lipomas and atypical lipomatous tumors, the latter otherwise referred to as well-differentiated liposarcomas. These amount to approximately 20% of all soft-tissue sarcomas.1 Although lipomas are generally considered to be easily distinguished from atypical lipomatous tumors by morphology, on the basis that the latter contain unequivocal atypical hyperchromatic nuclei often associated with bands of fibrosis, in some cases the differences may be subtle.2
A number of studies have shown that there is a strong correlation between the presence of supernumerary ring or giant marker chromosomes, derived from chromosome 12q13-15 where MDM2, CDK4, SAS, HMGIC and other genes are located, and the morphological features of atypical lipomatous tumors and dedifferentiated liposarcomas. These genetic abnormalities, originally reported using cytogenetics,3, 4 and subsequently by reverse transcription polymerase chain reaction,5, 6, 7 fluorescence in situ hybridization (FISH),8, 9, 10 and later with antibodies to MDM2 and CDK4,5, 8 demonstrated that MDM2 amplification occurs consistently in atypical lipomatous tumor in which CDK4 is also frequently amplified. In contrast, other genes in the chromosomal region 12q13-15 are less commonly associated with copy number gain11, 12 and in a small number of cases CDK4 amplification has been reported in the absence of MDM2 copy number gain.8, 13, 14 It is also reported that the supernumerary ring or giant marker chromosomes present in atypical lipomatous tumors rarely contain alpha 12 satellite sequences (alphoid 12), which represent the repeated DNA sequences that compose part of the centromere 12.14, 15, 16
The recent availability of the commercial MDM2 fluorescent probe made us consider the value of using the presence of MDM2 amplification as an ancillary diagnostic tool for distinguishing lipomas from ALT in our clinical service. Furthermore, the presence of MDM2 amplification may be valuable for stratifying patients for treatment in the future as targeted therapy against MDM2 is already being developed.17, 18 We report here on the correlation between histological diagnoses and MDM2 amplification in a large series of mature lipomatous tumors. Other soft-tissue tumors were also examined for MDM2 amplification for the purpose of assessing the specificity and sensitivity of MDM2 amplification as a diagnostic test.
Materials and Methods
A total of 443 tumors were selected between 2003 and 2008 by searching the diagnostic codes of the electronic files of the histopathology department of the Royal National Orthopaedic Hospital where the World Health Organization classification of tumors is used.16 This figure included 227 lipomatous tumors comprising lipoma, atypical lipomatous tumor, hibernoma, lipoblastoma, spindle cell and pleomorphic lipoma, dedifferentiated liposarcoma and pleomorphic liposarcoma (Table 1 ). Myxoid liposarcomas with the characteristic gene rearrangements, and lipomas <5 cm were not included in the study. Analysis of 201 non-lipomatous tumors was also included in the study (Table 2 ). The slides of these cases were reviewed by four histopathologists (TK, DD, RT, AMF) who were unaware of the previous diagnoses, and the site and size of the tumor, or if a tumor was recurrent, and a diagnostic consensus was reached. In all, 178 tumors diagnosed as either lipoma or atypical lipomatous tumors were diagnosed from 170 individuals, with a second (recurrent) tumor available from 8 patients. Overall, 150 of this tumor group (including primary and recurrent tumors) arose peripherally, and an additional 28 were sited in the abdominal or retroperitoneum. The study complies with the standards set out by the national research ethics committee (reference number 07/Q0506/8).
Fluorescence In Situ Hybridization
FISH using the ZytoLight SPEC MDM2/CEN12 Dual Color Probe kit (ZytoVision GmbH, Bremerhaven, Germany) was performed on a full tissue section, considered to be representative, of mature lipomatous tumors (lipomas and atypical lipomatous tumor), and on duplicate cores (1.0 mm) of non-lipomatous tumors. The tissue microarrays were made using a manual arrayer (Beecher Instrument, Sun Prairie, WI, USA). If the tumors were non-informative, the result was equivocal, and when alphoid signals were suspected, a full tissue section was analyzed.
The probe cocktail decorates the human chromosomal region MDM2 with a green signal, and the alpha satellite centromeric region of chromosome 12 (D12Z3 sequences) with a red signal. The centromere of chromosome 12 (centromere 12) is detected as a strong, intense red signal, whereas integrated alpha satellite 12 sequences (alphoid 12 signals) located at ring or marker chromosome are detected as faint signals compared with that of centromere 12.15 Confirmation of the alpha satellite 12 sequences was achieved by performing FISH using a Spectrum Green-labeled D12Z3 probe (Abbott Molecular, Des Plaines, IL, USA). CDK4 amplification was examined using the Poseidon CDK4 (2q13) & SE 12 probe kit (Kreatech, Amsterdam, The Netherlands), which contains CDK4 and D12Z3 probes. All FISH was performed according to the manufacturer's protocol.
Evaluation of the sampled tissue sections was carried out using fluorescence microscopy on an Olympus BX-50 microscope or on a Mirax viewer (3DHISTECH, Budapest, Hungary) following digitization of the slides using the Zeiss Mirax scanner. The signal number for MDM2 or CDK4, and centromere 12 was scored by counting a minimum of 50 non-overlapping nuclei per case, and the average number of MDM2 and centromere 12 signals, and CDK4 and centromere 12 signals, was then calculated. A ratio of >2.0 was considered to represent amplification (amplification-positive), a ratio of 2 was considered to be non-amplified (amplification-negative).9 Regardless of the MDM2:centromere 12 or CDK4:centromere 12 ratio, an average of 3 or more signals for centromere 12 was considered to represent copy number gain (referred by others previously as aneusomy or polysomy).9 All FISH was reviewed by at least two individuals experienced in interpreting FISH.
Immunohistochemistry
Immunohistochemistry was performed for CDK4 expression using a monoclonal antibody (clone DCS-31, Biosource International, Camarillo, CA, USA).8
Statistical Analysis
Unpaired t-test was used to correlate tumor size and MDM2 amplification status. Every analysis was two-sided and was performed using Prism version 4 for Macintosh (GraphPad Software, San Diego, CA, USA). P<0.05 was considered significant.
Results
MDM2 FISH on Lipomatous Neoplasms
Tables 1 and 2 provide summarized data generated from FISH analysis performed using the MDM2 and centromere 12 probe on a total of 428 tumors (227 lipomatous and 201 non-lipomatous). Six cases (1% of 434) were considered to be non-informative because of the absence of fluorescent signals and were omitted from the study. Of the 86 tumors classified histologically as lipomas, 84 (98%) contained the normal two copies of MDM2 (Figure 1a). MDM2 amplification was identified in the two remaining cases (2.3% of 86). On histological review the pathologists agreed that these tumors revealed low-grade nuclear atypia, and they were reclassified as atypical lipomatous tumors (Figure 1b).
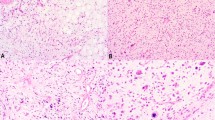
H&E and FISH of mature lipomatous tumors (lipoma and atypical lipomatous tumor) and high-grade liposarcomas. (a) A lipoma with typical features without MDM2 amplification. (b) H&E of a mature lipomatous tumor with low-grade atypia reclassified to atypical lipomatous tumor from lipoma on the basis of MDM2 amplification (FISH not shown). (c) An atypical lipomatous tumor with unequivocal atypia and MDM2 amplification. (d) H&E of an atypical lipomatous tumor with unequivocal atypia and with only scattered cells harboring high MDM2 copy number (FISH not shown). (e) High-grade component of a dedifferentiated liposarcoma (dedifferentiated liposarcoma) showing multiple tumor cells harboring high-level MDM2 amplification. (f) MDM2 amplification and multiple faint alphoid 12 signals in a tumor with unequivocal atypia. (g) FISH revealing multiple faint alphoid 12 signals, but no MDM2 amplification in the well-differentiated component of a dedifferentiated liposarcoma in which MDM2 was not detected (a, c in Table 1). (h) Pleomorphic liposarcoma with copy number gain of both centromere 12 and MDM2. Arrow and arrowhead indicate the strong bright signals of centromere 12 and multiple faint alphoid 12 signals, respectively. Bar, 50 μm (a–d) and 100 μm (g, h).
A total of 79 of 92 (86%) tumors classified histologically as atypical lipomatous tumors, revealed MDM2 amplification (Figure 1c, Table 1) as assessed using the strict definition of amplification described above. Seven of the MDM2 amplification-positive atypical lipomatous tumors (9% of 79) had low-level copy number (defined as tumors harboring on average number >10 MDM2 signals per nucleus),19 and these cases were indistinguishable morphologically from atypical lipomatous tumors with high copy number.
Dedifferentiated Liposarcomas (n=20)
Of the 92 atypical lipomatous tumors diagnosed on morphology alone, 14 (15%) had a dedifferentiated component and all but one, which occurred in the shoulder, arose de novo. The mature component of these 14 cases showed unequivocal nuclear atypia, thereby providing no difficulty for the pathologists reaching a consensus diagnosis of atypical lipomatous tumor. Six additional dedifferentiated liposarcomas, including one intra-abdominal or retroperitoneal tumor, of which the well-differentiated component was not available, were also analyzed by FISH. In total, the dedifferentiated component of 18 of 20 dedifferentiated liposarcomas showed MDM2 amplification (Figure 1e and Table 1). The well-differentiated components of the two MDM2 amplification-negative dedifferentiated liposarcomas were also negative for MDM2 amplification. The average signal ratio of MDM2:centromere 12 in the dedifferentiated liposarcomas was 1.77 fold greater (range 1.01 to 3.72) than in the corresponding well-differentiated component.
Correlation of MDM2 Copy Number Status in Primary and Recurrent Lipoma, Atypical Lipomatous Tumors
Of 20 recurrent tumors in the lipoma-atypical lipomatous tumor group (2 lipomas: thigh, buttock; 18 atypical lipomatous tumors diagnosed microscopically), there were 8 where both the primary and recurrent tumors were available for analysis, one of which involved the retroperitoneum and abdomen. The two lipomas (MDM2 amplification-negative) and five atypical lipomatous tumors (MDM2 amplification-positive) showed the same MDM2 amplification status in the primary and recurrent disease. One atypical lipomatous tumor, sited in the thigh and negative for MDM2 amplification in the primary neoplasm, revealed MDM2 amplification in the recurrent tumor.
Correlation of MDM2 Copy Number and Size of Lipoma, Atypical Lipomatous Tumor and Dedifferentiated Liposarcoma
Figure 2 shows the maximum dimensions of the primary peripherally sited lipoma-atypical lipomatous tumors (n=150) (intra-abdominal and retroperitoneal tumors were excluded). Of note is that all but 2 of the 60 MDM2 amplification-positive tumors were >10 cm; these 2 were intramuscular dedifferentiated liposarcoma (4 cm and 4.5 cm) and both occurred in the shoulder area. The two lipomas, which were reclassified as atypical lipomatous tumor on the basis of the MDM2 amplification, were >20 cm.
Correlation of the size of mature lipomatous tumors (lipoma and atypical lipomatous tumor) and dedifferentiated liposarcoma with MDM2 amplification status (n=150). A total of 90 MDM2 amplification-negative tumors included: ▴, dedifferentiated liposarcoma (n=1); ▪, dedifferentiated liposarcoma with multiple faint alphoid 12 signals (n=1); ⊕, atypical lipomatous tumor in which multiple copies of MDM2 was only detected after multiple slides were analyzed (n=1); ○, cases diagnosed as atypical lipomatous tumor without MDM2 amplification and were reclassified as lipoma (n=7). X, tumors with only scattered cells exhibiting multiple copies of MDM2 with only 2–3 copies of centromere 12 (n=3). A total of 60 MDM2 amplification-positive tumors included ▴, dedifferentiated liposarcoma (n=14); ▪, dedifferentiated liposarcoma with multiple faint alphoid 12 signals (n=1); □, atypical lipomatous tumor with multiple faint alphoid 12 signals (n=5). Of the 178 lipomas and atypical lipomatous tumor, 20 recurrent neoplasms and 8 retroperitoneal/abdominal tumors were omitted from this figure. The broken line depicts the average tumor dimension (SD). Numbers illustrate range in mm. P<0.001, all MDM2 amplification-negative cases vs all MDM2 amplification-positive case, using unpaired t-test.
Out of 90, 43 (48%) MDM2 amplification-negative peripherally sited tumors were >10 cm. The size of the 13 MDM2 amplification-negative lipomatous tumors originally classified as atypical lipomatous tumor ranged from 5.5 to 22 cm (Figure 2). The two recurrent lipomas (MDM amplification-negative in primary and recurrent lesions) first presented as 19 and 5.5 cm, and later recurred as 12 and 22 cm, respectively.
Atypical Lipomatous Tumor and Dedifferentiated Liposarcoma with Multiple Faint Alphoid 12 Signals
An unexpected finding was the presence of aggregates of multiple faint alphoid 12 signals in eight MDM2 amplification-positive atypical lipomatous tumors from six patients. Two of these had recurrent disease and in both, multiple faint alphoid 12 signals were present in the primary and recurrent tumors (Figure 1f, Table 3 , cases 1, 4). In addition, multiple faint alphoid 12 signals were detected in both components of two dedifferentiated liposarcomas. One of the dedifferentiated liposarcomas was MDM2 amplification-positive and the other MDM2 amplification-negative (Figure 3, Table 3, case 5 and 6, respectively). These multiple faint alphoid 12 signals, which were shown to represent multiple copies of D12Z3 sequences, could be distinguished from centromere 12 signals by the signal intensity of the latter (Figures 1f, g and 3).15
H&E and FISH of two dedifferentiated liposarcomas harboring multiple faint alphoid 12 signals: one with and one without MDM2 amplification. (a–f) A dedifferentiated liposarcoma with both MDM2 and CDK4 amplification and multiple faint alphoid 12 signals. (a) Low-power magnification showing the well-differentiated and the dedifferentiated component of a dedifferentiated liposarcoma. (b, c) High-power magnification of the well-differentiated and the dedifferentiated components in (a) respectively. (d) MDM2 FISH of the dedifferentiated component showing multiple faint alphoid 12 signals (red) and MDM2-amplification (green). (e) FISH using a SpectrumGreen-labeled D12G3 probe on the dedifferentiated component confirming the presence of multiple faint alphoid 12 signals, and no copy number gain of centromere 12. (f) CDK4 FISH on the dedifferentiated component showing multiple faint alphoid 12 signals (green) and CDK4 amplification (red). (g–l) A dedifferentiated liposarcoma harboring multiple faint alphoid 12 signals and CDK4 amplification in the absence of MDM2 amplification. (g, h) Microphotographs of the well-differentiated component and the dedifferentiated component, respectively. (i) MDM2 FISH of the dedifferentiated component showing multiple faint alphoid 12 signals (red) and absence of MDM2 amplification (green). (j, k) CDK4 showing nuclear immunoreactivity of tumor cells in the well-differentiated component and the dedifferentiated component, respectively. (l) CDK4 FISH of the dedifferentiated component, showing CDK4 amplification (red) and multiple faint alphoid 12 signals (green). Note the CDK4 signals are depicted as red and centromere 12 signals are detected by SpectrumGreen-labeled D12G3 probe in e, f and l. An arrow and an arrowhead indicate the strong bright centromere 12 signals and multiple faint alphoid 12 signals, respectively, Bar, 100 μm (a, g, h) and 50 μm (b, c, j, k).
In an attempt to explain the presence of the multiple faint alphoid signals, FISH and immunohistochemistry for CDK4 were performed on those cases with this finding. CDK4 amplification was identified in six of the eight tumors from six individuals: FISH in the remaining two cases were uninformative (Table 3, case 1 and 2). Six of the cases were also immunoreactive for CDK4, and the remaining two samples from one individual were uninformative (Table 3, case 1). Two of the eight cases were dedifferentiated liposarcomas, one with (Table 3, case 5, Figure 3a–f) and one without MDM2 amplification (Table 3, case 6, Figure 3g–l) both of which revealed CDK4 amplification and protein expression. The CDK4 amplification and immunoreactivity were seen in both the well-differentiated, and dedifferentiated components of these two tumors (Figure 3j and k).
It was noteworthy that the average signal ratio of CDK4:centromere 12 in the 2 dedifferentiated liposarcomas was 2.28 and 1.73 fold greater, and that the multiple faint alphoid 12 signals in the same tumors were 2.46 and 1.63 fold greater in the dedifferentiated liposarcoma components than in their respective well differentiated component parts (Table 3, case 5 and 6, respectively). This difference in signal ratio was similar to that observed in that of MDM2:centromere 12 in the dedifferentiated liposarcoma with MDM2 amplification (2.47 fold, Table 3, case 5).
Correlation of Clinicopathological Findings in Atypical Lipomatous Tumors Without MDM2 Amplification (n=13)
By applying the strict definition for identifying tumors with MDM2 amplification (see Materials and methods),9 we found that 14% (13 of 92) of tumors classified on histological grounds as atypical lipomatous tumor did not reveal MDM2 amplification. MDM2 amplification was then sought on an additional four slides in each of these cases, and a single slide from one tumor (12 cm in the thigh) revealed extensive MDM2 amplification. On more detailed analysis of the remaining 12 neoplasms, including study of copy number and protein expression of CDK4 by FISH and immunohistochemistry, respectively, we considered that six could be classified as atypical lipomatous tumor using robust criteria other than the detection of MDM2 amplification. Three of the cases revealed a very small number of cells with MDM2 amplification and therefore had not been classified as having MDM2 amplification because they did not fulfill the defined criteria. These tumors were lipoma-like atypical lipomatous tumors (Figure 1d) and did not show multiple faint alphoid 12 signals or harbor CDK4 amplification or immunoreactivity. Two other cases showed significant atypia and represented the well-differentiated component of dedifferentiated liposarcoma. One of the dedifferentiated components of these two tumors failed to show amplification of MDM2 or CDK4, multiple faint alphoid 12 signals and immunoreactivity for CDK4. In contrast, the other harbored multiple faint alphoid 12 signals and CDK4 amplification in both components (Figure 3g, Table 3, case 6). Lastly, one atypical lipomatous tumor did not have MDM2 amplification on the initial presentation but it was detected when it recurred. We consider that the evidence provided for these six (7% of 92) cases argues that these tumors are best classified as atypical lipomatous tumor without MDM2 amplification.
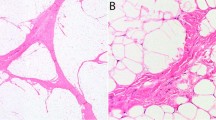
Of the remaining 6 of 12 mature lipomatous tumors diagnosed as atypical lipomatous tumor but which revealed neither MDM2 nor CDK4 amplification and/or immunoreactivity, extensive sclerosis with and without necrosis was present in three, and a significant inflammatory component was observed in two of the cases (Supplementary Figure S1). One case revealed metaplastic bone formation. On review it was considered that these six cases would have been better classified as lipomas. The data argue that MDM2 amplification is 93.4% sensitive for the diagnosis of atypical lipomatous tumor.
MDM2 Amplification in Lipomatous Tumors Other Than Lipomas and Atypical Lipomatous Tumors
None of the non-lipoma-atypical lipomatous tumors analyzed including hibernoma (n=13), lipoblastoma (n=5), spindle cell lipoma (n=15) and pleomorphic liposarcoma (n=9) demonstrated either MDM2 amplification or multiple faint alphoid 12 signals (Table 1). However, one pleomorphic liposarcoma showed copy number gain of both MDM2 and centromere 12 signals of the same intensity (Figure 1h).
MDM2 FISH on Non-Lipomatous Tumors (n=201)
The result of FISH on 201 neoplasms, which had not been diagnosed as lipomatous tumors, is shown in Table 2. We found that seven harbored MDM2 amplification, and one had multiple alphoid 12 signals and CDK4 amplification. The former included three spindle cell sarcoma, not otherwise specified (Figure 4a), two soft tissue osteosarcomas (Figure 4b), and 2 myxofibrosarcomas. On review of the clinical/radiological findings and histopathology of the seven cases, the diagnoses of the three ‘spindle cell sarcomas not otherwise specified’ were considered very likely to represent dedifferentiated liposarcoma as the diagnoses had been made on needle cores, and all three tumors were retroperitoneal or intra-abdominal (resected material not available for review). The two ‘soft-tissue osteosarcoma’ were also sited in the retroperitoneum, and having subsequently reviewed previous histology from other hospitals, we found that both revealed an atypical lipomatous tumor component. The diagnoses of these tumors were revised to dedifferentiated liposarcoma. Of the two ‘myxofibrosarcomas’ we found that one patient had been treated previously in another hospital (pathology not available for review) for a spindle cell retroperitoneal tumor. The second tumor (ankle, Figure 4c) on review was found to have a small area of mature fat with atypical features, which had previously been overlooked (Supplementary Figure S2). Hence, both of the MDM2 amplification-positive myxofibrosarcomas were considered to represent dedifferentiated liposarcoma.
H&E and FISH of non-lipomatous soft-tissue tumors. (ai, ii) H&E and FISH of a spindle cell sarcoma not otherwise specified with MDM2 amplification. (bi, ii) H&E and FISH of a soft-tissue osteosarcoma with MDM2 amplification, respectively. (ci, ii) H&E and FISH of a myxofibrosarcoma with MDM2 amplification, respectively. (di, ii) H&E and FISH of a myxofibrosarcoma with copy number gain of both centromere 12 and MDM2, respectively. (ei-ii) a myxofibrosarcoma with multiple faint alphoid 12 signals in the absence of MDM2 amplification. (eiii) FISH using a SpectrumGreen-labeled D12G3 probe confirming multiple faint alphoid 12 signals in the absence of copy number gain of centromere 12. (eiv) CDK4 FISH demonstrating CDK4 amplification and multiple faint alphoid 12 signals. Bar, 50 μm (ai, bi, ci, di) and 100 μm (ei). Note the CDK4 signals are depicted as red and centromere 12 signals are detected as green in eiii and eiv. An arrow and arrowhead indicate the strong bright signals of centromere 12 and faint alphoid 12 signals.
FISH analysis of non-lipomatous tumors without MDM2 amplification (Table 2) revealed that the remaining eight soft tissue osteosarcomas had copy number gain of centromere 12 but not MDM2 or CDK4 amplification, and that 6 of the remaining 43 myxofibrosarcomas showed copy number gain of both centromere 12 and MDM2 (Figure 4d). Only one tumor revealed multiple faint alphoid 12 signals and CDK4 amplification, in the absence of MDM2 amplification, and this had been diagnosed as a low-grade myxofibrosarcoma (4 cm maximum dimension, thigh) (Figure 4e, case 7 in Table 3). Following histological review it was considered more likely to represent an atypical lipomatous tumor with low-grade dedifferentiation, despite no well-differentiated component being identified, as the histology was considered not to be typical for a myxofibrosarcoma. Of the remaining non-lipomatous tumors (Table 2), we found that copy number gain of centromere 12 signals was detected in one high-grade leiomyosarcoma and in one pleomorphic sarcoma. Normal MDM2 copy number and centromere 12 number was found in the remaining 145 non-lipomatous tumors.
Discussion
Distinguishing lipomas from atypical lipomatous tumor on microscopy can be difficult2 and the presence of MDM2 amplification, identified by a variety of techniques, has been found to correlate strongly with histological findings in several studies.5, 6, 7, 8, 9, 10, 20 As a consequence, the identification of this molecular abnormality is advocated as a useful ancillary diagnostic marker, and can be considered 100% specific in the appropriate clinical and histological context.9
Our data support the findings of others and shows that there is a good correlation between microscopic diagnosis and MDM2 amplification status.5, 6, 7, 8, 9, 10 As in the study by Zhang et al10 we found that we had a tendency to ‘overcall’ lipomas, whereas the risk of ‘under-calling’ an atypical lipomatous tumor (MDM2 amplification-positive) was much less. Specifically, we found that 2 of 86 (2%) tumors reported as lipomas exhibited MDM2 amplification, whereas 79 of 92 (87%) of the mature lipomatous tumors diagnosed as atypical lipomatous tumor harbored MDM2 amplification.
As with the interpretation of all diagnostic markers, using the presence and absence of MDM2 amplification to classify a tumor as an atypical lipomatous tumor and a lipoma, respectively, must be interpreted in the light of other relevant clinicopathological information. The importance is highlighted by the knowledge that MDM2 amplification occurs in the majority of parosteal osteosarcomas and a small number of high-grade central osteosarcomas.21, 22, 23, 24 Nevertheless, the large numbers of soft-tissue sarcomas of various types studied here, and by others,3, 5, 8 argues that MDM2 amplification is largely restricted to atypical lipomatous tumor and dedifferentiated liposarcoma in soft-tissue tumors. Indeed, only 8 of 201 (4%) non-lipomatous tumors were found to harbor MDM2 amplification with or without multiple faint alphoid 12 signals. These cases had been reported as spindle cell sarcomas not otherwise specified (3 of 11), myxofibrosarcomas (3 of 43), and soft-tissue osteosarcomas (2 of 10). However, following review of the histology and the clinical histories, it was considered that all should be reclassified as dedifferentiated liposarcoma. These data underscore the need for always considering the diagnosis of dedifferentiated liposarcoma when reporting such tumors, and for meticulous macroscopic and microscopic analysis of tumors. The findings also demonstrate the importance of obtaining accurate clinical data, and of reviewing previous pathology.
It is interesting that 4% (8 of 201) of the non-lipomatous sarcomas analyzed in this study harbored MDM2 amplification or multiple faint alphoid 12 signals, and that this is almost identical to the proportion of pleomorphic malignant fibrous histiocytomas (6 of 159.4%) considered by Fletcher to represent dedifferentiated liposarcoma in a histological review, almost 20 years ago.25 The exploitation of technological advances and knowledge of molecular genetics, which allows better characterization of tumors, adds weight to the argument that many pleomorphic sarcomas can be classified to a particular lineage. It is also noteworthy that MDM2 amplification is reported previously in dedifferentiated liposarcoma with osteosarcomatous differentiation26, and that Evans noted that myxofibrosarcoma could be mistaken for dedifferentiated liposarcoma.27
Although the detection of a genetic abnormality in a tumor can be a valuable diagnostic tool, the absence of such a marker is always less informative and cannot be employed to exclude a diagnosis. With this in mind, we scrutinized the histology and the clinical information of the 13 tumors diagnosed microscopically as atypical lipomatous tumor in which the molecular hallmark MDM2 was not detected. The discrepancy in the histological classification and MDM2 amplification status in these cases is not explained by the presence of CDK4 amplification, another frequently amplified gene on the ring/marker chromosomes, which has been shown previously to occur occasionally in the absence of MDM2 amplification.8 It was therefore noteworthy that MDM2 amplification was present in a recurrent atypical lipomatous tumor and not in the primary neoplasm, that two of the atypical lipomatous tumors had a dedifferentiated component, and finally that unequivocal MDM2 amplification was identified in only very occasional cells in 3 of the 13 tumors, thereby excluding them from being classified as having MDM2 amplification when the strict definition of amplification is employed by us, and others.9 Finally, we found that MDM2 amplification was detected in only one of the five slides analyzed, highlighting that rarely MDM2 amplification may be localized in a mature lipomatous tumor. It is surprising that the above set of observations has not been reported previously but this may reflect that the occurrence of such events is rare. Hence, on the basis of our findings we consider that we provide sound evidence that 7 of 13 mature lipomatous tumors without MDM2 amplification would be best classified as atypical lipomatous tumor. The remaining six tumors classified as atypical lipomatous tumor without MDM2 amplification had significant amounts of inflammation, and or fibrosis and fat necrosis and on review were considered to have been ‘overcalled’. This highlights that these findings represent potential diagnostic pitfalls when reporting mature lipomatous tumors. When taking the above evidence together, we consider that MDM2 amplification is 93.5% sensitive for diagnosing atypical lipomatous tumor.
The finding that MDM2 amplification was found in very occasional tumor cells in three tumors, and in only one of the four slides in another case, raises important questions with respect to setting criteria for scoring a tumor as being amplification-‘positive’ for MDM2. Nevertheless, it is generally accepted that scoring systems are not perfect and arbitrary cutoff points are required for implementation of a useful diagnostic test. However, continued critical assessment of the diagnostic criteria that pathologists employ are required if a better understanding of a disease in its full spectrum is to be acquired. The findings are also of interest at a biological level as they raise the possibility that MDM2 amplification is not an initiating event in the development of these tumors. However, the fact that MDM2 amplification is generally observed throughout the tumors suggests that when this genetic event occurs the cells affected outgrow those without it.
Multiple faint alphoid 12 signals in the ring/giant chromosome were first reported, using cytogenetic techniques, in two mature lipomatous tumors, two osteosarcomas14 and subsequently in a dedifferentiated liposarcoma.15 They represent satellite DNA sequence of centromere 12, whereas other genes such as MDM2 and CDK4 derive from chromosome 12q13-15 in the ring/giant chromosome.28 To our knowledge, this abnormality has not been demonstrated unequivocally by FISH in atypical lipomatous tumors on formalin-fixed paraffin-embedded material previously. Our finding highlights the need for awareness of this chromosomal structural abnormality because it could be misinterpreted as gain of copy number of both centromere 12 and MDM2, which has occasionally been described in dedifferentiated liposarcoma,29, 30 but never in atypical lipomatous tumor.5, 8, 9, 10
Our study shows that 4% (4/92) of atypical lipomatous tumors and 10% of the dedifferentiated liposarcomas (2/20) as well as one low-grade myxofibrosarcoma revealed multiple faint alphoid 12 signals. All seven tumors with these signals also harbored CDK4 amplification regardless of the MDM2 amplification status. Of note was that in the two cases of dedifferentiated liposarcoma, the gain of multiple alphoid 12 signals was ∼ twofold greater in the dedifferentiated component compared with the respective well-differentiated areas, a finding mirrored in the greater number of MDM2/CDK4 copies in the high-grade component of dedifferentiated liposarcoma compared with their respective low-grade component. Although multiple faint alphoid 12 signals was found to be associated with aggressive behavior in other tumors,14, 15, 26 analysis of a larger cohort of patients is required to determine whether the presence of multiple faint alphoid 12 signals is associated with a significant risk of the tumor dedifferentiation.
In conclusion, this paper emphasizes the benefits and limitations of MDM2 as an adjunct in diagnosing mature lipomatous tumors. The work highlights that it is rare for a biological test to be entirely specific or sensitive for diagnostic purposes. Nevertheless, with MDM2 amplification reaching 94% sensitivity for the diagnosis of atypical lipomatous tumor, makes it a valuable diagnostic marker. Awareness of potential pitfalls of molecular pathology, and provision of diagnoses in conjunction with morphology and relevant clinical information will improve diagnostic accuracy even further.
References
Dei Tos AP . Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4:252–266.
Evans HL . Atypical lipomatous tumor, its variants, and its combined forms: a study of 61 cases, with a minimum follow-up of 10 years. Am J Surg Pathol 2007;31:1–14.
Fletcher CD, Akerman M, Dal Cin P, et al. Correlation between clinicopathological features and karyotype in lipomatous tumors. A report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. Am J Pathol 1996;148:623–630.
Bassett MD, Schuetze SM, Disteche C, et al. Deep-seated, well differentiated lipomatous tumors of the chest wall and extremities: the role of cytogenetics in classification and prognostication. Cancer 2005;103:409–416.
Binh MB, Sastre-Garau X, Guillou L, et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol 2005;29:1340–1347.
Hostein I, Pelmus M, Aurias A, et al. Evaluation of MDM2 and CDK4 amplification by real-time PCR on paraffin wax-embedded material: a potential tool for the diagnosis of atypical lipomatous tumours/well-differentiated liposarcomas. J Pathol 2004;202:95–102.
Shimada S, Ishizawa T, Ishizawa K, et al. The value of MDM2 and CDK4 amplification levels using real-time polymerase chain reaction for the differential diagnosis of liposarcomas and their histologic mimickers. Hum Pathol 2006;37:1123–1129.
Sirvent N, Coindre JM, Maire G, et al. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol 2007;31:1476–1489.
Weaver J, Downs-Kelly E, Goldblum JR, et al. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol 2008;21:943–949.
Zhang H, Erickson-Johnson M, Wang X, et al. Molecular testing for lipomatous tumors: critical analysis and test recommendations based on the analysis of 405 extremity-based tumors. Am J Surg Pathol 2010;34:1304–1311.
Gisselsson D, Hoglund M, Mertens F, et al. The structure and dynamics of ring chromosomes in human neoplastic and non-neoplastic cells. Hum Genet 1999;104:315–325.
Pedeutour F, Forus A, Coindre JM, et al. Structure of the supernumerary ring and giant rod chromosomes in adipose tissue tumors. Genes Chromosomes Cancer 1999;24:30–41.
Italiano A, Bianchini L, Keslair F, et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int J Cancer 2008;122:2233–2241.
Gisselsson D, Hoglund M, Mertens F, et al. Chromosomal organization of amplified chromosome 12 sequences in mesenchymal tumors detected by fluorescence in situ hybridization. Genes Chromosomes Cancer 1998;23:203–212.
Sirvent N, Forus A, Lescaut W, et al. Characterization of centromere alterations in liposarcomas. Genes Chromosomes Cancer 2000;29:117–129.
Fletcher C, Unni K, Mertens F . Tumours of Soft Tissue and Bone. IARC Press: Lyon, 2002.
Conyers R, Young S, Thomas DM . Liposarcoma: molecular genetics and therapeutics. Sarcoma 2011;2011:483154.
Singer S, Socci ND, Ambrosini G, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res 2007;67:6626–6636.
Weaver J, Goldblum JR, Turner S, et al. Detection of MDM2 gene amplification or protein expression distinguishes sclerosing mesenteritis and retroperitoneal fibrosis from inflammatory well-differentiated liposarcoma. Mod Pathol 2009;22:66–70.
Storlazzi CT, Mertens F, Domanski H, et al. Ring chromosomes and low-grade gene amplification in an atypical lipomatous tumor with minimal nuclear atypia. Int J Oncol 2003;23:67–71.
Gamberi G, Ragazzini P, Benassi MS, et al. Analysis of 12q13-15 genes in parosteal osteosarcoma. Clin Orthop Relat Res 2000;377:195–204.
Mejia-Guerrero S, Quejada M, Gokgoz N, et al. Characterization of the 12q15 MDM2 and 12q13-14 CDK4 amplicons and clinical correlations in osteosarcoma. Genes Chromosomes Cancer 2010;49:518–525.
Yoshida A, Ushiku T, Motoi T, et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol 2010;23:1279–1288.
Duhamel LA, Ye H, Halai D, et al. Frequency of mouse double minute 2 (MDM2) and mouse double minute 4 (MDM4) amplification in parosteal and conventional osteosarcoma subtypes. Histopathology 2012;60:357–359.
Fletcher CD . Pleomorphic malignant fibrous histiocytoma: fact or fiction? A critical reappraisal based on 159 tumors diagnosed as pleomorphic sarcoma. Am J Surg Pathol 1992;16:213–228.
Yoshida A, Ushiku T, Motoi T, et al. Well-differentiated liposarcoma with low-grade osteosarcomatous component: an underrecognized variant. Am J Surg Pathol 2010;34:1361–1366.
Evans HL . Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol 1979;3:507–523.
Marshall OJ, Chueh AC, Wong LH, Choo KH . Neocentromeres: new insights into centromere structure, disease development, and karyotype evolution. Am J Hum Genet 2008;82:261–282.
Segura-Sanchez J, Gonzalez-Campora R, Pareja-Megia MJ, et al. Chromosome-12 copy number alterations and MDM2, CDK4 and TP53 expression in soft tissue liposarcoma. Anticancer Res 2006;26 (6C):4937–4942.
Tsuji T, Fukuda T, Tamiya S, et al. Dedifferentiated components versus well-differentiated components in dedifferentiated liposarcoma: a comparative study of their proliferative activity and interphase cytogenetics using MIB-1 immunostaining and fluorescence in situ hybridization. Int J Surg Pathol 1999;7:1–10.
Acknowledgements
We thank Dr Rafael Malagoli for scanning the slides on to the Zeiss MIRAX scanner and Zytovison for supplying the ZytoLight SPEC MDM2/CEN12 Dual Color Probe kit. This work was funded by Skeletal Cancer Action Trust, UK, and was made possible by the existence of the Royal National Orthopaedic Hospital Musculoskeletal Biobank and Research Programme. ‘This work was supported by the UCL Experimental Cancer Medicine Centre, which is funded by Cancer Research UK and the NIHR. Part of this work was presented at the USCAP meeting 2009. The project has been supported by Skeletal Cancer Action Trust (SCAT), UK. ZytoLight SPEC MDM2/CEN12 Dual Color Probe kit was kindly provided as a gift by ZytoVision GmbH, Bremerhaven, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Rights and permissions
About this article
Cite this article
Kashima, T., Halai, D., Ye, H. et al. Sensitivity of MDM2 amplification and unexpected multiple faint alphoid 12 (alpha 12 satellite sequences) signals in atypical lipomatous tumor. Mod Pathol 25, 1384–1396 (2012). https://doi.org/10.1038/modpathol.2012.90
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.90
Keywords
This article is cited by
-
A scoring system combining clinical, radiological, and histopathological examinations for differential diagnosis between lipoma and atypical lipomatous tumor/well-differentiated liposarcoma
Scientific Reports (2022)
-
Total endoscopic left ventricle lipoma removal
Journal of Cardiothoracic Surgery (2021)
-
Qualitative evaluation of MRI features of lipoma and atypical lipomatous tumor: results from a multicenter study
Skeletal Radiology (2020)
-
Pilot study to differentiate lipoma from atypical lipomatous tumour/well-differentiated liposarcoma using MR radiomics-based texture analysis
Skeletal Radiology (2020)
-
Differentiating atypical lipomatous tumors from lipomas with magnetic resonance imaging: a comparison with MDM2 gene amplification status
BMC Cancer (2019)