Abstract
Cutaneous melanomas are characterized by a range of histological appearances, and several morphological variants have been described. In this study, we report a variant of superficial spreading melanoma that is characterized by large, irregular junctional melanocytic nests. The junctional nests varied in shape and size, showed focal tendency to confluence, and were often surrounded by a cuff of epidermal keratinocytes. The melanocytes comprising the nests showed variable cytological atypia. In most of the cases, scant intraepidermal or junctional single melanocytes were seen, and other well-documented diagnostic criteria for melanoma were lacking, and as a result, histological recognition of these tumors as melanoma was difficult. Some cases were associated with an invasive dermal component or showed evidence of sun damage. To provide supporting evidence for malignancy, we analyzed these tumors for genomic aberrations. Using array comparative genomic hybridization (aCGH), we identified multiple genomic aberrations in all analyzed cases. A similar pattern of genomic aberrations was seen in a control group of bona fide superficial spreading melanomas, suggesting that these ‘melanomas composed exclusively or predominantly of large nests’ are indeed variants of superficial spreading melanoma. Fluorescence in-situ hybridization (FISH) was positive in 40% of the cases. However, using aCGH, the FISH-negative cases showed multiple genomic aberrations in regions that are not covered by FISH. The low sensitivity of the FISH test can be explained by the fact that FISH only evaluates four genomic loci for aberrations, whereas aCGH surveys the entire genome. In summary, we present histological and molecular genetic evidence for a morphological variant of superficial spreading melanoma. Awareness of the histological features will aid in their correct diagnosis as melanoma, and in difficult cases, judicious application of ancillary tests such as aCGH (rather than FISH) will assist accurate diagnosis.
Similar content being viewed by others
Main
Cutaneous melanomas are characterized by a diverse range of histological appearances, and several morphological variants have been described. The most common subtypes in histological classification systems are superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma.1
Although histological evaluation is a reliable means of accurately classifying melanocytic tumors as benign (nevi) or malignant (melanoma), this distinction can be difficult in a minority of cases. Based on the fact that melanomas are characterized by chromosomal aberrations, molecular genetic tests such as comparative genomic hybridization (CGH)2, 3, 4 and fluorescence in situ hybridization5, 6, 7, 8 have been shown to be useful ancillary techniques, which assist accurate classification of melanocytic tumors. Multiple chromosomal gains and losses are identified with CGH in the majority (>95%) of melanomas, whereas melanocytic nevi typically lack chromosomal aberrations.3, 4 Therefore, identification of multiple chromosomal aberrations in a melanocytic tumor is a strong evidence in favor of the tumor being a melanoma.
In this study, we describe clinical, pathological, and molecular genetic features of a distinct morphological variant of superficial spreading melanoma, termed ‘melanomas composed exclusively or predominantly of large nests’ (MLN). These neoplasms are composed almost exclusively of large nests, and other well-documented diagnostic criteria for melanoma are lacking, and as a result, accurate histological recognition as melanoma may be challenging.
Materials and methods
Specimens and histological evaluation
The study was approved by the institutional ethics committee of the Medical University Graz and was conducted according to the principles set forth in the Declaration of Helsinki. All specimens were fixed in 4% buffered formalin, routinely processed, and embedded in paraffin. For histological evaluation, 4-μm-thick sections were stained routinely with hematoxylin and eosin. In addition, sections were stained immunohistochemically by the labeled streptavidin–biotin technique using anti-human melanosome (Clone HMB-45, 1:300, Dako, Glostrup, Denmark), anti-S-100 protein (polyclonal, 1:2000, Dako), anti-Human Melan-A (Clone A103, 1:50, Dako), anti-melanoma associated antigen (Clone NKI/C3, 1:200, BioGenex, San Ramon, CA, USA), pan-cytokeratin (clone MNF116, 1:500, Dako), Ki67 (clone SP6, 1:300, Dako), and MPM2 (clone O.T.181, 1:8000, Dako).
The first author (HK) initially identified cutaneous melanocytic tumors composed predominantly of large- and medium-sized melanocytic nests during his routine diagnostic work and informed the co-authors that these tumors might represent a subtype of superficial spreading melanoma. A study group was initiated, and over a period of several months, the co-authors prospectively collected melanocytic tumors that showed a similar histological appearance and large melanocytic nests during their routine diagnostic work.
The identified cases were sent to the Medical University Graz for genomic analysis and histological re-evaluation (Table 1). Cases were categorized as ‘melanomas composed exclusively or predominantly of large nests’ (MLN) if the junctional component of the tumors was composed almost exclusively of large melanocytic nests of variable shape and size. The melanocytes in the nests were often crowded, and showed variable degrees of cytological atypia, including nuclear enlargement and occasional mitotic figures. Suprabasilar spread of single melanocytes was not identified, but involvement of adnexal epithelium by junctional nests was focally identified. If the junctional component of the tumors was composed of large- to medium-sized melanocytic nests of variable size and shape, along with many intervening suprabasilar intraepidermal or junctional melanocytes exhibiting cytological atypia, the tumors were classified as conventional superficial spreading melanoma, according to well-documented histological criteria.9
DNA extraction and aCGH
We isolated DNA from formalin-fixed, paraffin-embedded tissue to perform a genome-wide, high-resolution analysis of chromosomal aberrations using an array-CGH (aCGH) technique. The 10-μm-thick, formalin-fixed, paraffin-embedded tissue sections were mounted on a PET membrane covered microscope slide (Zeiss, Göttingen, Germany). To obtain pure tumor DNA, melanocytic nests were laser-microdissected using a ‘PALM-Zeiss Laser Microdissection and Pressure Catapulting system’ (Zeiss). DNA was extracted from the laser-microdissected tissue using the Chemagic DNA Tissue Kit (Chemagen, Baesweiler, Germany) according to the manufacturer's instructions.
In three cases (case nos. 4, 17, and 19 in Table 1), <250 ng DNA was obtained and the isolated DNA was then amplified using a GenomePlex Single Cell Whole Genome Amplification Kit (#WGA4; Sigma-Aldrich, Hamburg, Germany). The amplification product was purified using the GenElute PCR Clean-up Kit according to the manufacturer's instructions (#NA1020; Sigma-Aldrich) and labeled for aCGH as described previously.10 As shown previously, no major artifacts were induced by whole-genome amplification and a similar signal-to-noise ratio of the aCGH profile was observed when using amplified or non-amplified DNA.11
aCGH was performed in all cases in which DNA was available. Briefly, 250 ng unamplified or amplified DNA was labeled using the Bioprime Array CGH Genomic Labeling Kit according to the manufacturer’s instructions (Invitrogen, Carlsberg, CA, USA). Test and reference DNA (Promega, Madison, WI, USA) were differentially labeled with dCTP-Cy5 and dCTP-Cy3, respectively (GE Healthcare, Piscataway, NJ, USA). Genome-wide analysis of DNA copy number changes was conducted using an oligonucleotide array containing 60 000 probes according to the manufacturer’s protocol version 6.0 (Agilent, Santa Clara, CA, USA). Slides were scanned using Agilent’s microarray scanner G2505B and analyzed using Agilent Feature Extraction and DNA Workbench software 6.5.018.
Fluorescence in situ hybridization
Cases in which material was available were studied with fluorescence in-situ hybridization (FISH) using probes targeting the following four loci (Abbott Molecular, Des Plaines, IL, USA): the probe for RREB1 at chromosome 6p25 was labeled with SpectrumRed; for MYB at chromosome 6q23 with SpectrumGold probe; for CCND1 at chromosome 11q13 with SpectrumGreen; and for the centromere of chromosome 6 with SpectrumAqua.5, 12 The 5-μm-thick sections were obtained from formalin-fixed paraffin-embedded tissue and mounted on SuperFrost Plus positively charged slides. The slides were baked overnight at 56 °C, deparaffinized in xylene, and dehydrated in ethanol. All tissue sections were pretreated with × 1 SSC for 35 min at 80 °C and digested for 15 min with Protease II following the instructions of the suppliers (Abbott Molecular). After a second dehydration step, the probes were applied to the sections and the covered slides were sealed with rubber cement, heat-denatured, and hybridized at 37 °C for 16 h. After washing in × 2 SSC/0.3% NP40 at 73 °C for 2 min, the slides were counterstained with DAPI I in mounting medium (1000 ng/ml, Abbott Molecular) and visualized under a Zeiss Axioplan microscope using a HBO103 lamp and the appropriate filters for the five fluorescent dyes. At least 30 nonoverlapping intact nuclei were assessed for the presence of signal gains and losses by a dermatopathologist (HK) and a molecular biologist (GP). The cutoff levels for the four probes were calculated as described by Gerami et al.5
Results
Clinical characteristics
Eleven MLNs were identified. They occurred equally in males and females, with a median age of 61 years (range 44–79 years). The majority of MLNs arose primarily on the limbs and trunk. Eleven of 16 conventional superficial spreading melanoma occurred in males, with a median age of 68 years (range 37–87 years).
The majority of MLN showed typical clinical features of melanoma, such as asymmetry of color and shape, irregular borders, multiple colors, and size greater than 6 mm. In addition to these clinical features, dermatoscopy showed several features characteristic of melanoma, such as asymmetry, multicomponent overall structure, irregular blotches, atypical pigment net, and large, irregular round to oval dots and globules (Figure 4a–c). These unusual large dots and globules appeared to correspond to the large nests seen in the histological sections. Some clinicians pointed out that in patients with multiple melanocytic lesions, the MLNs were very different from all other pigmented lesions, and raised clinical suspicion for melanoma (‘the ugly duckling sign’13).
Pathological features
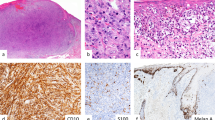
MLNs were composed of large, irregular junctional melanocytic nests of varying size and shape, which at low magnification yielded a striking ‘cannonball’ appearance to the junctional zone (Figures 1a, 2a, 3a, and 4d). At scanning power, the nests in MLNs were large, irregular in shape and size, and showed focal tendency to confluence (Figures 1b, 2b, and 4e). The nests were located predominantly at the tips and occasionally at the sides of epidermal rete ridges. In most cases, the nests were discrete and rounded, often with a cuff of epidermal keratinocytes surrounding them (Figure 1b and d). Occasionally, the nests bridged adjacent rete ridges or fused with adjacent nests resulting in an elongated oval outline (Figures 2b and 3a). The melanocytes comprising the nests showed moderate to marked cytological atypia (Figure 1c). The proliferation index in the melanocytes within the nests was low (Figure 1e). Some MLNs showed varying degrees of actinic elastosis.
MLN from the upper arm of a 49-year-old female (case 1). (a) Scanning magnification showing a sharply circumscribed melanocytic tumor composed of large, irregular junctional nests. The inset displays an unstained section after laser microdissection of the nests for DNA isolation and aCGH. (b) The junctional component of the tumors is composed almost exclusively of large melanocytic nests of variable size and shape. Intervening intraepidermal or junctional single melanocytes are rare. (c) The melanocytes comprising the nests show mild to moderate cytological atypia and prominent nucleoli. (d) Pan-cytokeratin (MNF-116) staining highlights the cuff of epidermal keratinocytes surrounding the large nests. (e) Ki-67 staining shows a low proliferation index in the melanocytes. (f) FISH (red: RREB1 at 6p25; orange: MYB at 6q23; green: CCND1 at 11q13; blue: centromere of chromosome 6) shows abnormal increase in the number of red signals. (g) aCGH shows partial losses of chromosome 3, 7, and 11 and a gain on chromosome 6 indicating chromosomal instability and providing biological evidence that this tumor is a melanoma.
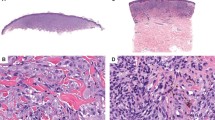
MLN from the upper arm of a 71-year-old male (case 3). (a) Small melanocytic tumor composed of large nests of variable size and shape. (b) Some nests show a tendency to confluence. (c) The aCGH profile shows partial losses of chromosome 3, 10, and 17. The commercially available melanoma FISH probe set, which covers only three loci on chromosome 6 (RREB1, CEP6, and MYB) and one locus on chromosome 11 (CCND1), was negative in this case.
MLN from the foot of a 79-year-old female (case 9). (a) The nests were located predominantly at the tips of epidermal rete ridges with a ‘cannonball’ appearance. Some nests bridged adjacent rete ridges. (b) Many intraepidermal or junctional melanocytes are seen between large melanocytic nests in the Melan-A stain. (c) The aCGH profile shows a gain of the whole chromosome 8, as well as a gain on chromosome 13 and a loss on chromosome 15.
MLN from the forearm of a 61-year-old male (case 6). (a–c) Dermatoscopic pictures showed an asymmetric overall structure, inhomogeneous brown areas, and numerous, variously sized globules and dots with various shades of brown. These globules and dots might represent the clinical correlate to the large nest in the histological section. Panel a represents the left upper part (insert: clinical picture), panel b the lower part, and panel c the right upper part of the lesion. (d) Large nests of variable size and shape. (e) Serial sections reveal confluence and formation of large, bulky nests. (f) Deeper sections showed an invasive component. No DNA for aCGH was available for this case.
In 7 of 11 cases, intraepidermal and junctional melanocytes were absent or very rare between the nests (Figures 1a and b, and 4d and e). The remaining four MLN showed intraepidermal and junctional melanocytes between the large nests (Figure 3b). The melanocytes in these cases were chiefly arrayed singly in a lentiginous distribution along the basal epidermal layer between the nests, but small numbers of suprabasilar intraepidermal melanocytes were also identified.
In 6 of 11 cases, the melanocytic proliferation was confined to the junctional zone, and in the other 5 cases, atypical melanocytes invaded the dermis. This dermal-invasive melanoma component was not identified in the initial section, but after several serial cuts (Figure 4d–f).
Conventional superficial spreading melanomas were composed of medium to large junctional melanocytic nests that varied considerably in size and shape, often with elongate or fusiform outlines. While some nests were surrounded by epidermal keratinocytes, often the keratinocytes surrounding nests were inapparent, particularly on the deep (dermal) aspect of the nests. Between the nests, numerous melanocytes were present in a lentiginous pattern as well as in a suprabasilar intraepidermal (pagetoid) distribution. The melanocytes within and between the nests showed moderate to marked cytological atypia and variable degrees of pigmentation.
Molecular genetic alterations
Eight of 11 MLN were analyzed with aCGH. All analyzed cases showed multiple chromosomal aberrations, including gains and losses of whole chromosomes or parts of chromosomes (Figures 1g, 2c, and 3c). Losses involving chromosome 3, 5, 7, 9, 12, 10, 11, 15, and 17 and gains involving chromosome 5, 6, 7, 8, 10, and 13 were most frequently observed. No characteristic chromosomal aberration was associated with the morphology of large nests. A very similar pattern of chromosomal aberrations was also observed in the group of conventional superficial spreading melanomas.
FISH was performed on 10 of 11 MLNs. Only 4 of these 10 cases were FISH-positive (Figure 1f). However, the FISH-negative cases showed multiple aberrations in aCGH in regions that were not covered by the FISH probes (Figure 2c). All MLN that were FISH-positive also showed aberrations in aCGH. The clinical and pathological characteristics, and the aCGH and FISH results are summarized in Table 1.
Discussion
A nested arrangement of melanocytes is a recurrent pattern in melanocytic tumors, including acquired nevi, dysplastic nevi, and melanoma. In acquired nevi, the nests are generally uniform, are populated by non-atypical melanocytes, and lack significant confluence of adjacent nests.14 In conventional superficial spreading melanoma, junctional nests are often highly variable in shape and size, are usually composed of highly atypical melanocytes, and show a tendency to confluence. In addition, suprabasilar intraepidermal (pagetoid) spread of melanocytes are often observed.15 Dysplastic nevi share some histopathological features with superficial spreading melanoma, but show lesser degrees of cytoarchitectural atypia and lack significant suprabasilar pagetoid spread of melanocytes.14
Although MLNs and conventional superficial spreading melanoma exhibit similar clear-cut malignant genomic profiles in aCGH (discussed below), MLNs differed histologically from conventional superficial spreading melanomas in having a predominant arrangement of melanocytes in very large nests. This presentation with large, rounded (‘cannonball’) junctional nests with varying size and shape and often only few melanocytes in a lentiginous and suprabasilar distribution in the intervening epidermis may pose problems in accurate histopathological recognition of these tumors as melanoma. Typical diagnostic criteria for superficial spreading melanoma such as solitary melanocytes predominating over melanocytes arranged in nests, melanocytes scattered through all epidermal layers, and lack of sharp lateral circumscription are lacking in MLN. The most characteristic histopathological criteria for diagnosis of MLNs are asymmetry, large melanocytic nests with marked variation in size and shape, confluence of the nests, melanocytes with marked cytological atypia, and associated actinic elastosis in some cases.
Genomic instability is a hallmark of human cancers16 and is particularly so in melanomas.3, 4 Benign melanocytic tumors do not show any chromosomal aberrations, but over 95% of melanomas show a high level of genomic instability manifested by chromosomal aberrations that affect most commonly chromosomes 1, 6, 7, 9, 10, and 11.3 All of the tumors in this study showed multiple gains and losses of several chromosomes. The pattern of chromosomal aberrations included multiple gains and losses of whole chromosomes and chromosomal parts, as well as breaks within chromosomes suggesting structural rearrangements within the genome. The same pattern of chromosomal aberrations is well described as being characteristic of melanoma (especially melanoma arising on intermittently, non-chronic sun-exposed skin),2, 17 and was also observed in the control group of conventional superficial spreading melanoma. These observations support the existence of pronounced genomic instability in MLN, and provide biological evidence of their malignant nature. Taken together with the morphological appearances, these data support the thesis that MLN are variants of superficial spreading melanoma.
The 4-probe FISH test has been reported to have a sensitivity and specificity of 85–90% for the diagnosis of melanoma,5, 6 but is of limited utility in the assessment of histologically ambiguous melanocytic skin tumors.12 Although the aCGH profiles correlated well with the morphological features of MLN, the correlation with FISH was poorer. The higher sensitivity of aCGH is explained by the fact that the FISH test only evaluates four genomic loci for gains and losses, in contrast to aCGH, which surveys the entire genome (Figure 2c).
Six of our 11 MLN cases represented in situ melanoma, and therefore the prognosis in these cases is expected to be excellent. The presence of a dermal-invasive component in five MLNs provides additional evidence for their malignant potential, and suggests that they may be characterized by in situ and invasive phases, analogous to conventional superficial spreading melanoma. Although we do not have follow-up evidence showing metastatic potential of these five cases of invasive MLN, the presence of multiple genomic aberrations detectable by aCGH demonstrates beyond reasonable doubt that these are bona fide melanomas. However, a recent study suggested that FISH-negative melanomas may have a better prognosis than FISH-positive tumors.18 As most MLN were FISH-negative, one might speculate that MLNs might have also a favorable prognosis. Studies of larger numbers of cases are required to test this hypothesis.
From a diagnostic point of view, two issues might be noteworthy. First, most of the MLN were correctly diagnosed as malignant melanomas by clinicians on the basis of clinical criteria (ABCDE rules, dermatoscopy, and ugly ducking sign), while the histological appearances presented difficulties in definitive diagnosis as melanoma. Second, in almost half of our cases, an invasive dermal component appeared in serial cuts, although the primary section only showed large, irregular junctional nests. Therefore, pathologists encountering predominantly nested atypical melanocytic tumors (particularly when accompanied by a clinical suspicion of melanoma) should perform deeper sections in order to maximize the chances of detecting an invasive melanoma component and should consider ancillary tests such as aCGH to confirm the diagnosis in difficult cases. Close communication with the clinician and correlation of the clinical, dermatoscopic, and pathological findings is critical to ensure accurate diagnosis.
In conclusion, we have presented morphological and molecular genetic evidence for a rare morphological variant of superficial spreading melanoma. The unusual appearance together with the absence of some classically described histopathological criteria for melanoma renders accurate histological diagnosis of these tumors difficult. Awareness of the existence and features of this peculiar presentation of melanoma will aid their recognition. In cases in which doubt exists after conventional histological examination, aCGH can serve as a useful ancillary test to confirm the diagnosis.
Change history
01 August 2012
An Erratum to this paper has been published: https://doi.org/10.1038/modpathol.2012.67
References
de Vries E, Bray F, Coebergh JW, et al. Malignant melanoma: introduction. In: LeBoit PE, Burg G, Weedon D, Sarasin A (eds) Pathology and Genetics of Skin Tumours 1st edn. IARC Press: Lyon, France, 2006, pp 52–65.
Bastian BC, LeBoit PE, Hamm H, et al. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res 1998;58:2170–2175.
Bastian BC, Olshen AB, LeBoit PE, et al. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol 2003;163:1765–1770.
Bauer J, Bastian BC . Distinguishing melanocytic nevi from melanoma by DNA copy number changes: comparative genomic hybridization as a research and diagnostic tool. Dermatol Ther 2006;19:40–49.
Gerami P, Jewell SS, Morrison LE, et al. Fluorescence in situ hybridization (FISH) as an ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg Pathol 2009;33:1146–1156.
Vergier B, Prochazkova-Carlotti M, de la Fouchardiere A, et al. Fluorescence in situ hybridization, a diagnostic aid in ambiguous melanocytic tumors: European study of 113 cases. Mod Pathol 2011;24:613–623.
Newman MD, Lertsburapa T, Mirzabeigi M, et al. Fluorescence in situ hybridization as a tool for microstaging in malignant melanoma. Mod Pathol 2009;22:989–995.
Newman MD, Mirzabeigi M, Gerami P . Chromosomal copy number changes supporting the classification of lentiginous junctional melanoma of the elderly as a subtype of melanoma. Mod Pathol 2009;22:1258–1262.
Haneke E, Bastian B . Superficial spreading melanoma. In: LeBoit PE, Burg G, Weedon D, Sarasin A (eds) Pathology and Genetics of Skin Tumours 1st edn. IARC Press: Lyon, France, 2006, pp 66–67.
Wiesner T, Obenauf AC, Cota C, et al. Alterations of the cell-cycle inhibitors p27(KIP1) and p16(INK4a) are frequent in blastic plasmacytoid dendritic cell neoplasms. J Invest Dermatol 2010;130:1152–1157.
Geigl JB, Obenauf AC, Waldispuehl-Geigl J, et al. Identification of small gains and losses in single cells after whole genome amplification on tiling oligo arrays. Nucleic Acids Res 2009;37:e105.
Gaiser T, Kutzner H, Palmedo G, et al. Classifying ambiguous melanocytic lesions with FISH and correlation with clinical long-term follow up. Mod Pathol 2010;23:413–419.
Grob JJ, Bonerandi JJ . The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol 1998;134:103–104.
Barnhill RL . Melanocytic nevi and tumor progression: perspectives concerning histomorphology, melanoma risk and molecular genetics. Dermatology 1993;187:86–90.
McGovern VJ, Cochran AJ, Van der Esch EP, et al. The classification of malignant melanoma, its histological reporting and registration: a revision of the 1972 Sydney classification. Pathology 1986;18:12–21.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011;144:646–674.
Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–2147.
North JP, Vetto JT, Murali R, et al. Assessment of copy number status of chromosomes 6 and 11 by FISH provides independent prognostic information in primary melanoma. Am J Surg Pathol 2011;35:1146–1150.
Acknowledgements
This work was funded by grants from the Jubilaeumsfonds of the Oesterreichische Nationalbank (13837) (to LC), the Harry J. Lloyd Charitable Trust (to RM) and the Lucille Castori Center for Microbes, Inflammation and Cancer, Memorial Sloan-Kettering Cancer Center (to RM).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kutzner, H., Metzler, G., Argenyi, Z. et al. Histological and genetic evidence for a variant of superficial spreading melanoma composed predominantly of large nests. Mod Pathol 25, 838–845 (2012). https://doi.org/10.1038/modpathol.2012.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.35
Keywords
This article is cited by
-
Nested melanoma
Modern Pathology (2013)
-
Malignant dermatofibroma: clinicopathological, immunohistochemical, and molecular analysis of seven cases
Modern Pathology (2013)