Abstract
Nephrogenic adenoma is a benign lesion of the urinary tract, particularly the urinary bladder. It is a gross and microscopic mimicker of urothelial neoplasm or metastatic carcinoma. Several histological patterns (tubular, tubulocystic, polypoid, papillary, fibromyxoid) have been recognized, but a flat pattern has not been described. Histologically, nephrogenic adenoma consists of tubules, cysts or papillae lined by flat to polygonal cells with frequent hobnail appearance. The stroma is often edematous or has a granulation tissue-like appearance with acute or chronic inflammation. By immunohistochemistry, nephrogenic adenomas are positive for renal epithelial markers CK7, CD10 and alpha-methylacyl-coenzyme A racemase, and negative for bladder urothelium or prostate markers. Recent studies have shown that nephrogenic adenomas are positive for PAX2 and PAX8. We encountered an interesting case of tubular nephrogenic adenoma with adjacent areas suspicious of flat urothelial atypia. Immunohistochemistry for PAX2 and PAX8 were positive in these areas, unveiling a flat pattern of nephrogenic adenoma. This case prompted us to study 15 cases of nephrogenic adenoma to determine additional instances of flat pattern and to assess the value of PAX2 and PAX8 immunoreactivity to diagnose nephrogenic adenoma. PAX2 and PAX8 immunostaining was positive in 14/15 and 15/15 cases, respectively. The flat pattern was present at least focally adjacent to tubular, polypoid and papillary areas, in 8/15 cases of nephrogenic adenoma. In conclusion, the flat pattern is a common finding in nephrogenic adenomas, but easily under recognized by morphologic examination and may be confused with flat urothelial lesions with atypia. Immunostains for PAX2 and PAX8 are useful in the detection of nephrogenic adenomas and particularly unveil those nephrogenic adenomas with flat pattern.
Similar content being viewed by others
Main
Nephrogenic adenoma is a benign lesion of the urinary tract, most commonly found in the urinary bladder. It occurs more frequently in males and its prevalence is higher in kidney transplant recipients.1
Nephrogenic adenoma is a gross and microscopic mimicker of malignant lesions. Microscopically, nephrogenic adenomas are typically composed of tubules, cysts or papillae lined by flat to polygonal cells with a large nucleus and middle-sized nucleolus supported by loose fibrous stroma with different amounts of acute or chronic inflammation.2, 3 By immunohistochemistry, nephrogenic adenomas are positive for the renal tubule markers cytokeratin 7 (CK7), CD10, alpha-methylacyl-coenzyme A racemase (AMACR), PAX2 and PAX8, but negative for bladder urothelium or prostate antigens.4, 5, 6, 7, 8 The transcription factors PAX2 and PAX8 (paired box genes) are crucial proteins during the embryonic and fetal development of several organs, particularly intermediate mesoderm and Müllerian-derived tissues, and are expressed in nearly 100% of nephrogenic adenomas in some series.9, 10, 11, 12 Tong et al13 have shown that urothelial cells from the upper genitourinary tract are PAX8 positive, whereas urinary bladder urothelium lacks PAX8 expression.
Several histological patterns of nephrogenic adenoma have been described, including a traditional tubular, tubulocystic, polypoid and papillary. In 2007, Hansel et al14 described a new fibromyxoid variant of nephrogenic adenoma that may mimic mucinous adenocarcinoma. However, to our knowledge, a flat pattern accompanying these other patterns has not been reported to date.
During a routine sign-out session, we encountered a case of nephrogenic adenoma with tubular pattern that also showed an adjacent area suspicious for flat urothelial atypia. However, the cytologic features in this area were similar to those seen in the tubular nephrogenic adenoma. Immunohistochemistry for PAX2 and PAX8 was positive in the area suspicious for flat urothelial atypia, unveiling a flat pattern of nephrogenic adenoma. This case prompted us to study a total of 15 cases of nephrogenic adenoma to determine whether there were additional instances of flat pattern as a component of the usual tubular, polypoid and papillary nephrogenic adenoma variants, and assess the value of PAX2 and PAX8 immunoreactivity for the diagnosis of flat nephrogenic adenoma pattern.
Materials and methods
Case Selection
Fifteen bladder biopsies with the diagnosis of nephrogenic adenoma were evaluated under a protocol approved by the Institutional Review Board of The Methodist Hospital Research Institute. The biopsies, from the surgical pathology archives of the Department of Pathology and Genomic Medicine, The Methodist Hospital, Houston, TX, were collected from patients from 2009 through 2011.
Histology and Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue was sectioned at 4 μm thickness and stained with hematoxylin and eosin (H&E) for routine histologic diagnosis and characterization of the nephrogenic adenomas. For further evaluation, immunohistochemistry was performed using a Ventana Benchmark automated stainer (Ventana, Tucson, AZ). Antigen retrieval was carried out using steam heat. Primary antibodies against the following proteins were used: PAX2 (clone PAX2, 1:50 dilution, ZYMED, Grand Island, NY); PAX8 (polyclonal, 1:30 dilution, Proteintech, Chicago, IL); thrombomodulin (clone 1009, 1:10 dilution, DAKO, Carpinteria, CA); and p63 (clone 4A4, prediluted, Ventana). Appropriate positive and negative controls were run in parallel. Histological and immunohistochemical evaluation of the cases was performed by the authors (SPO and JYR).
Results
The results are summarized in Table 1.
Clinical Data
Eleven patients were male (73%) and four were female (27%). The age ranged from 21 to 82 years with a median of 71 years old. In eight cases, a bladder biopsy was obtained for the presence of hematuria and a history of bladder tumor. The biopsies of the remaining seven cases were performed on each patient for urethral stricture, bladder neck contracture, hydronephrosis with stent replacement, benign prostatic hyperplasia, surveillance after a kidney transplant, chronic cystitis and bladder reconstruction for Prune belly syndrome. None of the patients had a previous diagnosis of nephrogenic adenoma. No diagnosis of recurrent nephrogenic adenoma has been established in subsequent biopsies from any of these cases.
Histopathology
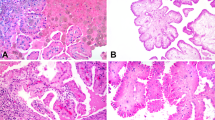
From all 15 cases of nephrogenic adenoma, 8 had a solely tubular pattern, 2 were pure polypoid, 2 showed tubular and polypoid components and 3 were papillary. Notably, 8 cases (53%) showed adjacent areas suspicious for flat urothelial atypia, ranging from focal to extensive involvement. We suspected these areas represented a flat pattern of nephrogenic adenoma that extended into the adjacent urothelium (Figure 1). The flat pattern was composed mostly of one occasionally up to three layers of polygonal cells, some of them with a hobnail profile that abruptly interrupted the continuum of the urothelial mucosa (Figure 2). In cases of nephrogenic adenoma with a tubular pattern, some of the tubules extended focally into the above urothelium. Focal connection of the surface component with underlying tubules was established after examination of multiple levels (Figures 2 and 3). The flat pattern varied in length and was patchy in some instances (Figure 2). No specific, previously described nephrogenic adenoma pattern was associated with the flat pattern.
Tubular nephrogenic adenoma with associated flat pattern. (a) Low magnification shows a stratified epithelium contiguous with nephrogenic adenoma (top panel, arrow, × 2) that could be interpreted as flat atypical urothelium. At higher magnification (middle panel, × 10), this epithelium is composed of cuboidal cells with a hobnail profile. Umbrella cells are not seen, and there are areas with a single layer of cells similar to those seen in the tubular nephrogenic adenoma (bottom panel, × 40). These are not architectural and/or cytological features of normal urothelium. (b) Immunostain for PAX8 highlights the tubular nephrogenic adenoma cells and the flat epithelial lining (top × 2, middle × 10 and bottom × 40). An area lined by urothelium in the same slide is negative for PAX8 (middle panel, inset, × 20). LP, lamina propria; MP, muscularis proper.
Polypoid nephrogenic adenoma with associated flat pattern. (a) At low magnification ( × 10), this polypoid nephrogenic adenoma shows areas with a flat component between urothelial mucosa (arrows). (b) There is a sharp transition from urothelium to the single layer of nephrogenic adenoma cells. (c) Immunohistochemistry for PAX2 is positive in the flat nephrogenic adenoma component and highlights the sharp transition of urothelium from nephrogenic adenoma cells as well as underlying tubular nephrogenic adenoma structures found in the lamina propria—not readily appreciated in the H&E stain. (d) In another case, a PAX8 immunostain highlights the extension of nephrogenic adenoma from the lamina propria into the surface (arrow) with the presence of the nephrogenic adenoma flat pattern. Normal urothelium is negative for PAX2 and PAX8 (c, d).
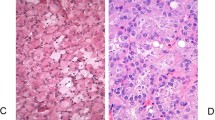
(a) The flat pattern in this tubular nephrogenic adenoma shows the sharp transition between the nephrogenic adenoma epithelium and the normal adjacent urothelium (arrow). Immunohistochemistry for PAX8 (b) and PAX2 (c) highlights the nephrogenic adenoma cells. Direct contact of nephrogenic adenoma cells to urothelium is also seen (b, thick arrow). (d) Thrombomodulin is negative in nephrogenic adenoma, but positive in urothelium and endothelial cells.
Immunohistochemistry
Fourteen of 15 cases (93%) were positive for PAX2 and all 15 cases (100%) were positive for PAX8, demonstrating a robust nuclear staining. Immunostainings for PAX2 and PAX8 highlighted the presence of the flat pattern that was adjacent to the usual tubular or papillary patterns of nephrogenic adenoma, which corresponded to the same areas suspicious of flat urothelial atypia identified on the H&E stain (Figures 1, 2, 3). Fourteen cases (93%) expressed both PAX2 and PAX8 with no particular correlation to the presence or absence of the flat pattern. In addition, PAX2 and PAX8 highlighted scattered nephrogenic adenoma cells that showed pagetoid spread in the urothelium in 3 cases of nephrogenic adenoma with the flat pattern (Figure 4). On the other hand, only one case of nephrogenic adenoma showed focal and patchy positivity for thrombomodulin and scattered cells positive for p63 (not shown). In all cases, normal urothelial lining cells adjacent to the flat pattern were strongly positive for p63 (nuclear) and thrombomodulin (cytoplasmic and membrane), and negative for both PAX2 and PAX8.
Pagetoid spread of nephrogenic adenoma cells within urothelium in areas adjacent to flat nephrogenic adenoma pattern. (a) A PAX2 immunostain highlights nephrogenic adenoma cells with focal spread in a pagetoid fashion into the urothelial mucosa (arrow). Three cases of nephrogenic adenoma with the associated flat pattern showed this feature. (b) Thrombomodulin is positive in urothelial cells, but negative in the same cluster of nephrogenic adenoma cells with pagetoid spread seen on panel a (arrow).
Discussion
The term ‘nephrogenic adenoma’ was first coined by Friedman and Kuhlenbeck in 195015 after they described a series of ‘adenomatoid’ tumors of the bladder that resembled renal tubular structures ‘…such as collecting tubules and Henle’s loop.’15
Historically, there was controversy regarding the origin of nephrogenic adenomas reflected in several theories that had been postulated since its original description. Some theories suggest that the bladder urothelium develops nephrogenic metaplastic changes after urologic manipulation and persistent chronic inflammation. Another theory suggests that nephrogenic adenomas may represent mesonephric embryologic remnants. Most recently, in 2002, Mazal et al16 demonstrated that nephrogenic adenomas arising in post-transplant kidney patients showed an identical chromosomal status to the kidney donor genetic profile, even when the transplant came from the opposite gender. This evidence supports the concept that nephrogenic adenomas arise from desquamated renal epithelial cells that implant in the bladder mucosa at least in the setting of kidney transplantation.
Although nephrogenic adenomas are considered benign lesions, they may recur in up to 30–80% of cases.2, 3 However, recognition of this lesion is crucial since nephrogenic adenoma cannot only present as a urothelial neoplasm cystoscopically, but may also be misdiagnosed microscopically as invasive urothelial carcinoma with glandular differentiation or microcystic variant, signet ring cell carcinoma and clear cell carcinoma with hobnail cells as well as metastatic prostate carcinoma or renal cell carcinoma. Therefore, familiarity with the different growth patterns, cytologic features and characteristic stroma of nephrogenic adenoma is important. In some instances, immunohistochemistry is needed for confirmatory diagnosis. Histologically, nephrogenic adenomas consist of tubules, cysts, or papillae lined by flat, cuboidal, or polygonal cells, usually with a hobnail profile. The cells have a large nucleus and medium-sized nucleolus. Tubules or cysts of nephrogenic adenoma usually have a thickened basement membrane without stromal response. They are localized to the lamina propria and do not extend to or beyond the muscularis propria. The stroma is often edematous or has a granulation tissue-like appearance with acute or chronic inflammation. Several histological patterns of nephrogenic adenoma have been described, including tubular, tubulocystic, polypoid and papillary patterns. Recently, a fibromyxoid variant has also been recognized.14 Although papillary nephrogenic adenomas are located in the mucosal surface of the bladder and nephrogenic adenoma cells line the surface, to our knowledge, the finding of nephrogenic adenoma extending into adjacent urothelium as a ‘flat pattern’ has not been described or emphasized. In this study, we showed that the presence of a flat pattern in nephrogenic adenoma is not uncommon and often mixed with the more traditional tubular, polypoid, and papillary components.
The immunohistochemistry profile of nephrogenic adenoma shows characteristic expression of renal tubule markers CK7, CD10, AMACR, PAX2 and PAX8, and the absence of urothelial markers (thrombomodulin and p63) and prostate antigens (prostate-specific antigen (PSA) and prostate alkaline phosphatase (PAP)).4, 5, 6, 7, 8 Several studies have shown that PAX2 and PAX8 are expressed in nearly 100% of nephrogenic adenomas9, 10, 11, 12 and are helpful markers when the diagnosis of nephrogenic adenoma is difficult. In this study, we corroborated the frequent expression of PAX2 (93%) and PAX8 (100%) in our series of nephrogenic adenomas and identified a flat pattern associated with other traditional nephrogenic adenoma patterns that extended along the adjacent urothelium. The areas of transition between the nephrogenic adenoma epithelium and the urothelium were sharply demarcated in all cases (Figures 2 and 3) and, in three cases, we observed focal pagetoid spread of PAX2/PAX8-positive cells within the urothelium (Figure 4). However, pagetoid spread could also represent the extension of small tubules of nephrogenic adenoma into the surface.
Although normal and neoplastic urothelium from the renal pelvis and ureter can show immunoreactivity to PAX8,13 benign, dysplastic and neoplastic urothelium from the bladder lacks PAX8 expression.10, 13 Detection of PAX8 in the flat pattern of our series of nephrogenic adenomas (all located in the bladder) confirms a true flat growth of nephrogenic adenoma epithelium along the bladder mucosa instead of reactive, atypical or dysplastic urothelial cells adjacent to the nephrogenic adenoma.
The significance of a flat pattern of nephrogenic adenoma is uncertain. However, we hypothesize that the frequent presence of this flat pattern may be an important factor related to the high recurrence rate of nephrogenic adenomas (30–80%).2, 3 As most nephrogenic adenomas are detected as a small (up to 1 cm) incidental lesion via cystoscopy, identification of the true extent of nephrogenic adenoma, which may assume a flat pattern growth at its periphery contiguous with the adjacent urothelium, is practically impossible. Thus, fragments of flat nephrogenic adenoma may remain in the bladder after transurethral resection and possibly give rise to recurrences. The associated postresection inflammatory response might also have a role in the regrowth of the lesion. On the other hand, four of our cases had clinical histories suggestive of underlying chronic inflammation, which may favor the development of nephrogenic adenomas in our series as a reactive metaplastic process. Recurrence in this scenario may not necessarily account on the presence of a flat pattern of nephrogenic adenoma but to an ongoing metaplasia that could occur after additional instrumentation and/or inflammatory processes.
Cheng et al17 have shown that atypical nephrogenic metaplasia is a benign process that requires awareness to prevent erroneous diagnosis of malignancy. The possibility of either biopsying only the flat growth in an otherwise traditional nephrogenic adenoma, or biopsying an incidental nephrogenic adenoma composed solely of the flat pattern, could potentially be confused with flat urothelial lesions with atypia. This possibility should alert the pathologist to consider nephrogenic adenoma before rendering a diagnosis of urothelial atypia, and to use immunohistochemical stains such as PAX2 and PAX8 in conjunction with other urothelial dysplastic markers, such as CK20, CD44 and p53, to confirm the diagnosis.18 Also, it may be advisable to utilize PAX8 immunostaining with nephrogenic adenomas to evaluate the marginal status. However, this recommendation awaits further detailed study.
In this study, we described the presence of a flat pattern of nephrogenic adenoma associated with other traditional patterns (tubular, polypoid, papillary), first identified by routine H&E stains as suspicious for flat urothelial atypia, but later confirmed by PAX2 and PAX8 immunohistochemistry as flat nephrogenic adenoma. Focal spread following a pagetoid fashion into the urothelium was observed in three cases. Recognition of the true extent of a nephrogenic adenoma, which may assume a flat pattern growth, is practically impossible by cystoscopy. We hypothesize that incomplete removal of this flat nephrogenic adenoma on transurethral resection may have a role in local recurrence, a common clinical feature of nephrogenic adenomas.
References
Banyai-Falger S, Maier U, Susani M et al High incidence of nephrogenic adenoma of the bladder after renal transplantation. Transplantation 1998;65:511–514.
Amin W, Parwani AV . Nephrogenic adenoma. Pathol Res Pract 2010;206:659–662.
Rahemtullah A, Oliva E . Nephrogenic adenoma: an update on an innocuous but troublesome entity. Adv Anat Pathol 2006;13:247–255.
Fromont G, Barcat L, Gaudin J et al Revisiting the immunophenotype of nephrogenic adenoma. Am J Surg Pathol 2009;33:1654–1658.
Gupta A, Wang HL, Policarpio-Nicolas ML et al Expression of alpha-methylacyl-coenzyme A racemase in nephrogenic adenoma. Am J Surg Pathol 2004;28:1224–1229.
Herlitz LC, Tong GX, Hamele-Bena D et al Nephrogenic adenoma identified on urine cytology using PAX-2 immunostaining. Diagn Cytopathol 2008;36:47–49.
Pierre-Louis ML, Kovi J, Jackson A et al Nephrogenic adenoma: a light and electron microscopic and immunohistochemical study. J Natl Med Assoc 1985;77:201–205.
Skinnider BF, Oliva E, Young RH et al Expression of alpha-methylacyl-CoA racemase (P504S) in nephrogenic adenoma: a significant immunohistochemical pitfall compounding the differential diagnosis with prostatic adenocarcinoma. Am J Surg Pathol 2004;28:701–705.
Ozcan A, Liles N, Coffey D et al PAX2 and PAX8 expression in primary and metastatic mullerian epithelial tumors: a comprehensive comparison. Am J Surg Pathol 2011;35:1837–1847.
Ozcan A, Shen SS, Hamilton C et al PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol 2011;24:751–764.
Tong GX, Melamed J, Mansukhani M et al PAX2: a reliable marker for nephrogenic adenoma. Mod Pathol 2006;19:356–363.
Tong GX, Weeden EM, Hamele-Bena D et al Expression of PAX8 in nephrogenic adenoma and clear cell adenocarcinoma of the lower urinary tract: evidence of related histogenesis? Am J Surg Pathol 2008;32:1380–1387.
Tong GX, Yu WM, Beaubier NT et al Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol 2009;22:1218–1227.
Hansel DE, Nadasdy T, Epstein JI . Fibromyxoid nephrogenic adenoma: a newly recognized variant mimicking mucinous adenocarcinoma. Am J Surg Pathol 2007;31:1231–1237.
Friedman NB, Kuhlenbeck H . Adenomatoid tumors of the bladder reproducing renal structures (nephrogenic adenomas). J Urol 1950;64:657–670.
Mazal PR, Schaufler R, Altenhuber-Muller R et al Derivation of nephrogenic adenomas from renal tubular cells in kidney-transplant recipients. N Engl J Med 2002;347:653–659.
Cheng L, Cheville JC, Sebo TJ et al Atypical nephrogenic metaplasia of the urinary tract: a precursor lesion? Cancer 2000;88:853–861.
Oliva E, Pinheiro NF, Heney NM et al Immunohistochemistry as an adjunct in the differential diagnosis of radiation-induced atypia versus urothelial carcinoma in situ of the bladder: a study of 45 cases. Hum Pathol advance online publication, 27 November 2012 (E-pub ahead of print).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Piña-Oviedo, S., Shen, S., Truong, L. et al. Flat pattern of nephrogenic adenoma: previously unrecognized pattern unveiled using PAX2 and PAX8 immunohistochemistry. Mod Pathol 26, 792–798 (2013). https://doi.org/10.1038/modpathol.2012.239
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.239
Keywords
This article is cited by
-
Nephrogenic metaplasia in a female person with spinal cord injury: chronic irritation caused by Foley balloon was aggravated by a large uterine fibroid pressing upon the bladder wall for several years: a case report
Spinal Cord Series and Cases (2022)
-
Dataset for the reporting of urinary tract carcinoma—biopsy and transurethral resection specimen: recommendations from the International Collaboration on Cancer Reporting (ICCR)
Modern Pathology (2020)
-
Nephrogenic adenoma of the urinary tract: clinical, histological, and immunohistochemical characteristics
Virchows Archiv (2013)