Abstract
Liposarcomas are a heterogenous group of fat-derived sarcomas, and surgery with or without chemoradiation therapy remains the main stay of treatment. NY-ESO-1 is a cancer-testis antigen expressed in various cancers where it can induce both cellular and humoral immunity. Immunotherapy has shown promise in clinical trials involving NY-ESO-1-expressing tumors. Gene expression studies have shown upregulation of the gene for NY-ESO-1, CTAG1B, in myxoid and round cell liposarcomas. Herein, we evaluated the expression of NY-ESO-1 among liposarcoma subtypes by quantitative real-time PCR, western blot analysis, and immunohistochemistry. Frozen tissue for quantitative real-time PCR and western blot analysis was obtained for the following liposarcoma subtypes (n=15): myxoid and round cell (n=8); well-differentiated (n=4), and dedifferentiated (n=3). Formalin-fixed paraffin-embedded blocks were obtained for the following liposarcoma subtypes (n=44): myxoid and round cell (n=18); well-differentiated (n=10); dedifferentiated (n=10); and pleomorphic (n=6). Full sections were stained with monoclonal antibody NY-ESO-1, and staining was assessed for intensity (1−3+), percentage of tumor positivity, and location. In all, 7/8 (88%) and 16/18 (89%) myxoid and round cell expressed CTAG1B and NY-ESO-1 by quantitative real-time PCR and immunohistochemistry, respectively. Western blot correlated with mRNA expression levels. By immunohistochemistry, 94% (15/16) of positive cases stained homogenously with 2−3+ intensity. Also, 3/6 (50%) pleomorphic liposarcomas demonstrated a range of staining: 1+ intensity in 50% of cells; 2+ intensity in 5% of cells; and 3+ intensity in 90% of cells. One case of dedifferentiated liposarcoma showed strong, diffuse staining (3+ intensity in 75% of cells). Our study shows that both CTAG1B mRNA and protein are overexpressed with high frequency in myxoid and round cell liposarcoma, enabling the potential use of targeted immunotherapy in the treatment of this malignancy.
Similar content being viewed by others
Main
Liposarcomas, which account for approximately 11% of soft tissue malignancies, are a heterogenous group of fat-derived soft tissue sarcomas composed of neoplasms with differing histomorphology, cytogenetic and molecular abnormalities, and biological behavior.1 The subtypes include the more commonly encountered well-differentiated, myxoid and round cell, and dedifferentiated liposarcomas and the less commonly seen spindle cell and pleomorphic liposarcomas. Surgery with or without chemoradiation therapy remains the main stay of treatment. Unfortunately, tumor recurrence, progression, and metastasis remain a challenge and are largely dependent on subtype and tumor location.2
Cancer-testis antigens are a unique family of antigens, which have restricted expression to testicular germ cells in a normal adult but are aberrantly expressed on a variety of solid tumors, including soft tissue sarcomas, melanoma, and epithelial cancers.3, 4 Cancer-testis antigens are highly immunogenic and can elicit spontaneous T-cell and/or humoral responses upon exposure.3 Among the known cancer-testis antigens, NY-ESO-1 is considered the most immunogenic and has become an attractive target for cancer vaccine development, which would utilize the immune system to selectively target and eliminate the cancer-testis antigen-expressing tumor cells.3
Similar to other sarcomas, gene expression studies have reported the presence of multiple cancer-testis antigens in liposarcomas, including SSX, LAGE-1, CTAG1B, CT-7, CT-10, GAGE, BAGE, PRAME, and various MAGE antigens.4, 5, 6 Furthermore, Segal et al. (2005)7 reported increased CTAG1B and PRAME gene transcripts specifically in the myxoid and round cell subtype, which constitutes approximately one-third of all liposarcomas.1, 7 Other than a recent report of increased NY-ESO-1 protein expression in the myxoid and round cell subtype, NY-ESO-1 expression has only been minimally evaluated in liposarcomas.4, 8, 9
NY-ESO-1 expression was initially identified in a case of esophageal squamous cell carcinoma and has subsequently been described in a variety of malignancies, including melanoma, sarcomas, and in various additional carcinomas.10, 11 Protein expression in normal adult tissue appears to be restricted to the testis, more specifically spermatogonia.8 Studies have demonstrated high immunogenicity with the induction of both humoral and specific CD8+ cytotoxic T-cell immune responses against the NY-ESO-1-expressing tumors.12, 13, 14
Given the previous reports of increased NY-ESO-1 in liposarcomas, we sought to further characterize NY-ESO-1 expression in the various liposarcoma subtypes using quantitative real-time PCR, western blot analysis, and immunohistochemistry.
Material and methods
Case Material
The liposarcomas (n=44) evaluated by immunohistochemistry were retrospectively identified from 2003 to 2011 from the surgical pathology archives at The Ohio State University Wexner Medical Center and included the following subtypes: well-differentiated (n=10), myxoid and round cell (n=18), dedifferentiated (n=10), and pleomorphic (n=6). The cases were re-reviewed by a bone and soft tissue pathologist (OHI), and the diagnoses were confirmed per established morphological diagnostic criteria.15 Notably, regarding the myxoid and round cell liposarcomas, molecular studies, including fluorescent in situ hybridization and/or conventional cytogenetic analysis, performed on 12 of the cases demonstrated rearrangement of CHOP (DDIT3) gene at 12q13 and t(12;16)(q13;p11), respectively, confirming the diagnosis in those cases. The six tumors diagnosed as pleomorphic liposarcoma were histologically consistent with conventional pleomorphic liposarcoma. None of the six cases of the pleomorphic liposarcoma contained any areas of conventional myxoid and round cell liposarcoma akin to the pleomorphic myxoid liposarcoma variant from the criteria as established by Alaggio et al.16 A representative formalin-fixed, paraffin-embedded block was obtained for each tumor and submitted for NY-ESO-1 immunohistochemical staining. Furthermore, frozen tissue samples of additional cases (total n=15) of well-differentiated (n=4), myxoid/round cell (n=8), and dedifferentiated (n=3) liposarcomas were obtained for quantitative real-time PCR and western blot studies. Both paraffin-embedded and frozen tumor samples were obtained after approval through the Institutional Review Board Ohio State University, Department of Pathology (Institutional Review Board-approved protocol number-2002H0089).
RNA Extraction and Quantitative Real-Time PCR
RNA was extracted from frozen sarcoma samples using Ribozol (Amresco) and a modified manufacturer’s protocol for RNA extraction using Trizol reagent (Ambion). RNA was quantitated using a NanoDrop-ND 1000. One microgram of RNA per sample was reverse transcribed using the iScript cDNA synthesis kit (Biorad). Quantitative real-time PCR was performed in 10 ml reactions according to recommended conditions by Applied Biosystems. Taqman probes for GAPDH (Hs00266705_1) and CTAG1B (Hs00265824_m1), which encodes for NY-ESO-1, were used.17 Each sample was measured in triplicate. No template controls and no reverse transcriptase controls for each sample were included. Cycle threshold values were averaged across triplicate samples. GAPDH was used to calculate percentage relative expression of each sample. Samples were further normalized to the expression of the testis positive control. Standard deviations were calculated by comparisons of delta cycle thresholds for each well in a triplicate.
Western Blot
Protein was isolated from frozen sarcoma samples using Ripa buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCL, 1% NP40, 0.10% SDS) with protease inhibitor and was quantitated using Quant-iT protein assays (Invitrogen Life Technologies). Forty micrograms of protein per sample was run by electrophoresis on 10% gels and transferred. Antibodies and dilutions were NY-ESO-1 (08-312, 1:2500, Upstate Cell Signaling Solutions) and GAPDH (sc-32233, 1:200, Santa Cruz). Anti-mouse secondary was used for both antibodies (115-035-003, 1:10 000, Jackson ImmunoResearch). GAPDH was used for loading normalization. Radiographs were digitally scanned and quantified by densitometry using ImageJ software (http://rsbweb.nih.gov/ij/index.html).
Immunohistochemistry
NY-ESO-1, clone E978 (Santa Cruz Biotechnology; Santa Cruz, CA), was titered on normal testis, and testis was used as control tissue. Paraffin-embedded tissue was cut at 4 microns, placed in a 60 °C oven for 1 h, cooled, and then deparaffinized and rehydrated through xylenes and graded ethanol solutions to water. All slides were quenched for 5 min in a 3% hydrogen peroxide solution in water to block for endogenous peroxidase. Antigen retrieval was performed by heat-induced epitope retrieval, in which the slides were placed in a 1 × solution of Target Retrieval Solution (Dako, Carpinteria, CA) for 25 min at 96 °C using a vegetable steamer and cooled for 15 min in the solution. Slides were then placed on a Dako Autostainer Immunostaining System. The primary antibody NY-ESO-1 was diluted with a Dako antibody diluent at 1:100 and incubated for 60 min. Slides were then blocked for endogenous biotin using the Dako Biotin Blocking System. The secondary antibody, a 1:200 dilution of biotinylated goat anti- mouse (Vector, Burlingame, CA) prepared with Vector 2% Normal Goat Serum in Dako antibody diluent, was incubated for 30 min at room temperature. The detection system used was Vectastain Elite (Vector) for 30 min. Staining was visualized with the DAB+ chromogen (Dako; 5-min development). Slides were then counterstained in Richard Allen hematoxylin, dehydrated through graded ethanol solutions.
Quantitation of Immunohistochemistry
NY-ESO-1 immunopositivity was scored semiquantitatively for the percentage of tumor cells staining (0%; <5%; 5–<25%; 25–<50%; 50–<75%; and ≥75%), intensity (0, negative; 1+, weak staining/trace; 2+, moderate staining; and 3+, strong staining), and subcellular location (cytoplasmic and/or nuclear).
Results
Quantitative Real-Time PCR
We performed quantitative real-time PCR on a total of 15 liposarcoma samples, which included eight myxoid and round cell, three dedifferentiated, and four well-differentiated liposarcomas, as well as one testis sample as a positive control for CTAG1B expression. The results are depicted in Figure 1 and Table 3. Seven (88%) myxoid and round cell liposarcomas showed expression of CTAG1B, five of which were significantly higher than expression found in the testis. One (33%) dedifferentiated liposarcoma showed expression, but it was roughly one-tenth the expression level observed in the testis. None of the well-differentiated liposarcomas were positive for CTAG1B expression.
Relative fold CTAG1B expression. CTAG1B expression of liposarcoma samples was measured by quantitative real-time PCR. CTAG1B expression was normalized to GAPDH. Expression is plotted as fold expression relative to CTAG1B expression in testes. Only samples showing CTAG1B expression are plotted. One of the three dedifferentiated samples showed expression and none of the four well-differentiated liposarcomas showed expression. Seven of the eight myxoid/round cell liposarcoma samples were positive for CTAG1B expression; five of those at levels significantly higher than the testis sample. DDL; dedifferentiated liposarcoma; MRCL, myxoid and round cell liposarcoma.
Western Blot
We performed western blot analysis on the eight liposarcoma samples that demonstrated CTAG1B expression with quantitative real-time PCR, consisting of seven myxoid and round cell liposarcomas and one dedifferentiated liposarcoma. One testis sample was utilized as a positive control for NY-ESO-1 protein expression. The results are depicted in Figure 2 and Table 3. Four of the seven myxoid and round cell liposarcomas demonstrated NY-ESO-1 protein expression. The samples that contained low levels of CTAG1B by quantitative real-time PCR (two myxoid and round cell liposarcomas and one dedifferentiated liposarcoma) were all negative for protein expression on western blot analysis. Notably, the samples did not show even loading, with the testis demonstrating the highest total protein levels. However, after normalization to GAPDH expression, four of the five myxoid and round cell samples that had shown higher expression of CTAG1B mRNA by quantitative real-time PCR also had higher NY-ESO-1 protein expression by western blot.
Western blot analysis of NY-ESO-1. The seven myxoid/round cell liposarcomas and one dedifferentiated liposarcoma that showed CTAG1B expression via quantitative real-time PCR were analyzed for NY-ESO-1 protein expression by western blot as well as the testis control sample. A GAPDH loading control is shown. MRCL, myxoid/round cell liposarcoma; DDL, dedifferentiated liposarcoma; *, MRCL samples that showed very low expression of CTAG1B by quantitative real-time PCR.
Immunohistochemistry
The results of NY-ESO-1 immunohistochemical staining in liposarcomas are summarized in Tables 1, 2, and 3. Consistent immunoreactivity was noted in myxoid and round cell liposarcomas (89%) and to a lesser degree in pleomorphic (50%) and dedifferentiated liposarcomas (10%). The staining pattern was cytoplasmic and nuclear in all positive instances; however, a cytoplasmic predominance was apparent. All 10 cases of well-differentiated liposarcomas were negative for NY-ESO-1.
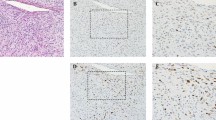
Of the 16 immunoreactive cases of myxoid and round cell liposarcoma, 15 (94%) demonstrated strong, homogenous immunoreactivity, as defined by >50% tumor cell positivity (Figure 3a); thus, only one tumor showed heterogeneous staining, characterized as 1+ intensity in 20% of tumor cells. Notably, 11 of the positive tumors showed 3+ intensity in ≥90% of tumor cells. The myxoid and round cell components demonstrated similar patterns of immunoreactivity in positive cases. Two tumors (11%) were negative for NY-ESO-1.
NY-ESO-1 immunoreactivity in liposarcoma subtypes. Diffuse, homogeneous NY-ESO-1 immunoreactivity in (a) myxoid/round cell and (b) pleomorphic liposarcomas (inset, H&E). (c) Diffuse NY-ESO-1 immunoreactivity with variable staining intensity in dedifferentiated liposarcoma (inset, H&E). All figures × 200.
The three NY-ESO-1-positive cases of pleomorphic liposarcoma demonstrated variable staining characteristics as follows: diffuse (90% of tumor cells) with 3+ staining intensity (Figure 3b); patchy (50% of tumor cells) with 1+ staining intensity; and focal (5% of tumor cells) with 2+ staining intensity. The single case of NY-ESO-1 immunoreactivity in dedifferentiated liposarcoma demonstrated diffuse staining (75% of tumor cells) with variable staining intensity (1 to 3+) (Figure 3c).
In addition, the case of myxoid and round cell liposarcoma that did not demonstrate CTAG1B mRNA by quantitative real-time PCR underwent formalin processing for further evaluation by H&E and immunohistochemical staining. The presence of myxoid and round cell liposarcoma was confirmed and subsequent staining for NY-ESO-1 showed strong, diffuse tumor immunoreactivity (3+ intensity of 95% of tumor cells).
Discussion
NY-ESO-1, a cancer-testis antigen, is a promising target for cancer immunotherapy, given its limited extra-tumoral expression and its characteristic high immunogenicity. CTAG1B, the gene for NY-ESO-1, is located at Xq28 and codes for an intracellular, 18-kDa protein.10, 17 The function of NY-ESO-1 is unknown; however, it has been postulated that cancer-testis antigens in general are involved in germ cell self-renewal or differentiation, conferring qualities such as immortality, self renewal, migratory ability, and capacity to transform to cancer cells upon expression.11, 18, 19
NY-ESO-1 was initially identified in esophageal squamous cell carcinoma utilizing ‘SERological identification of antigens by recombinant Expression cloning’ (SEREX) methodology.10 Subsequently, protein expression has been described in a variety of malignancies, including melanoma, neuroblastoma, lung, breast, prostate, endometrial, ovarian, uterine, hepatocellular, and bladder carcinomas.11 Increased protein expression in a variety of soft tissue sarcomas has also been reported, including synovial sarcomas, gastrointestinal stromal tumors, uterine leiomyosarcomas, angiosarcoma, malignant fibrous histiocytoma, and liposarcomas.4, 9, 20 The most common tumors to demonstrate NY-ESO-1 expression, as determined by immunohistochemistry with the monoclonal antibodies ES121 and E978, are myxoid and round cell liposarcoma (100%), neuroblastoma (82%), synovial sarcoma (80%), melanoma (46%), and epithelial ovarian cancer (43%).9, 20, 21, 22, 23 Studies involving NY-ESO-1-expressing tumors have demonstrated high immunogenicity with induction of both humoral and specific CD8+ cytotoxic T-cell immune responses.9, 12, 13, 14 Immunotherapy targeting this antigen has shown some promise in clinical trials involving NY-ESO-1-expressing tumors especially in synovial sarcomas and malignant melanomas.24, 25, 26, 27
In our study, we demonstrated that NY-ESO-1 is highly expressed in myxoid and round cell liposarcoma, which is a commonly encountered liposarcoma subtype. CTAG1B mRNA is consistently increased in these tumors (seven of eight samples tested), of which, five cases demonstrated greater levels than that seen in normal testis. Furthermore, the increased mRNA levels correlate with increased protein expression, as determined by western blot. Immunoreactivity by immunohistochemistry was demonstrated in 89% (16/18) of the myxoid and round cell liposarcomas evaluated, and, importantly, was distributed homogeneously in 94% of the positive cases. Fluorescent in situ hybridization analysis of the two negative cases of myxoid and round cell liposarcomas was consistent with rearrangement involving CHOP gene at 12q13, confirming the diagnosis in both cases. Interestingly, the myxoid and round cell sample that was negative for CTAG1B mRNA expression by quantitative real-time PCR demonstrated strong, homogenous immunoreactivity by immunohistochemistry. The reason for this discrepancy is unclear, but the GAPDH normalization control for this sample showed very low levels of expression on multiple trials, suggesting low quality or abundance of mRNA for the sample. As different antibodies were used for the immunohistochemical and western blot analyses, it is also possible that epitope variance could explain these results.
Our data parallels a recently published study that reported frequently homogeneous, NY-ESO-1 expression in 100% (25/25) myxoid and round cell liposarcomas evaluated by immunohistochemistry and quantitative real-time PCR.9 They did not see NY-ESO-1 immunoreactivity in any of the seven non-myxoid liposarcomas studied. On the contrary, we noted NY-ESO-1 expression in both pleomorphic (3/6; 50%) and dedifferentiated (1/10; 10%) subtypes. The overall staining distribution was more heterogeneous in the pleomorphic subtype compared with the myxoid and round cell subtype of liposarcoma. Notably, fluorescent in situ hybridization analysis performed on one of the three NY-ESO-1-positive pleomorphic liposarcomas was negative for rearrangements of CHOP (12q13) and EWSR1 (11q12) genes. Regarding dedifferentiated liposarcoma, we reported a low level of CTAG1B mRNA expression in a single case, which did not correlate with protein expression on subsequent western blot analysis. Immunohistochemistry also identified a case of NY-ESO-1 immunoreactivity, characterized as diffuse staining of variable intensity. Overall, it appears that there is an infrequent protein expression in this subtype. CTAG1B mRNA and NY-ESO-1 protein expression were not seen in any of the samples of well-differentiated liposarcoma evaluated by quantitative real-time PCR and immunohistochemistry respectively.
Tumor expression patterns of cancer-testis antigens is highly associated with DNA methylation status.28, 29 In a study of epithelial ovarian cancers utilizing microdissection techniques, heterogeneous NY-ESO-1 expression correlated with intra-tumoral differences in promoter as well as global DNA methylation status.29 Accordingly, a heterogenous pattern of expression might attenuate clinical and immunological responses to cancer-testis antigen-directed immunotherapy, which relies on antigen presentation. Thus far, studies have demonstrated a predominantly heterogeneous staining pattern with NY-ESO-1, with only infrequent homogenous staining seen within a particular tumor type.8, 21, 23 Other than myxoid and round cell liposarcomas, the only exception is in synovial sarcomas, where NY-ESO-1 expression was reported to be consistently homogenous, being present in 70% of positive tumors.20 A subsequent clinical trial utilizing adoptive immunotherapy targeting NY-ESO-1 has shown promise in synovial sarcomas.27
In conclusion, we report consistent, homogenous NY-ESO-1 expression within myxoid and round cell liposarcomas using quantitative real-time PCR, western blot analysis, and immunohistochemistry. These findings allow for the rational utilization of immunotherapy in this relatively common subset of liposarcomas, especially in the setting of metastatic disease or in patients who have failed or cannot tolerate conventional therapies.
References
Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: an analysis of 26,758 cases. Int J Cancer 2006;119:2922–2930.
Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg 2003;238:358–371.
Scanlan MJ, Gure AO, Jungbluth AA, et al. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 2002;188:22–32.
Ayyoub M, Taub RN, Keohan M-L, et al. The frequent expression of cancer/testis antigens provides opportunities for immunotherapeutic targeting of sarcoma. Cancer Immun 2004;4:7.
Skubitz KM, Cheng EY, Clohisy DR, et al. Differential gene expression in liposarcoma, lipoma, and adipose tissue. Cancer Invest 2005;23:105–118.
Skubitz KM, Pambuccian S, Manivel JC, et al. Identification of heterogeneity among soft tissue sarcomas by gene expression profiles from different tumors. J Transl Med 2008;6:23.
Segal NH, Blachere NE, Guevara-Patiño JA, et al. Identification of cancer-testis genes expressed by melanoma and soft tissue sarcoma using bioinformatics. Cancer Immun 2005;5:2.
Jungbluth AA, Chen YT, Stockert E, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer 2001;92:856–860.
Pollack SM, Jungbluth AA, Hoch BL, et al. NY-ESO-1 is a ubiquitous immunotherapeutic target antigen for patients with myxoid/round cell liposarcoma [published ahead of print February 22, 2012]. 2012) Cancer doi:10:1002/cncr.27446.
Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A 1997;94:1914–1918.
Nicholaou T, Ebert L, Davis ID, et al. Directions in the immune targeting of cancer: lessons learned from the cancer-testis Ag NY-ESO-1. Immunol Cell Biol 2006;84:303–317.
Jäger E, Chen YT, Drijfhout JW, et al. Simultaneous humoral and cellular response again cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med 1998;187:265–270.
Stockert E, Jäger E, Chen YT, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med 1998;187:1349–1354.
Jäger E, Nagata Y, Gnjatic S, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA 2000;97:4760–4765.
Weiss SW, Goldblum JR . Liposarcoma In:Enzinger and Weiss’s Soft Tissue Tumors 5th edn. Mosby Elsevier Inc: Philadelphia; 2008, pp 477–516.
Alaggio R, Coffin CM, Weiss SW, et al. Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol 2009;33:645–658.
Chen YT, Boyer AD, Viars CS, et al. Genomic cloning and localization of CTAG, a gene encoding an autoimmunogenic cancer-testis antigen NY-ESO-1, to human chromosome Xq28. Cytogenet Cell Genet 1997;79:237–240.
Zendman AJ, Ruiter DJ, Van Muijen GN. . Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol 2003;194:272–288.
Cronwright G, Le Blanc K, Götherström C, et al. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res 2005;65:2207–2215.
Jungbluth AA, Antonescu CR, Busam KJ, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer 2001;94:252–256.
Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res 2003;63:6076–6083.
Rodolfo M, Luksch R, Stockert E, et al. Antigen-specific immunity in neuroblastoma patients: antibody and T-cell recognition of NY-ESO-1 tumor antigen. Cancer Res 2003;63:6948–6955.
Barrow C, Browning J, MacGregor D, et al. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res 2006;12:764–771.
Jäger E, Gnjatic S, Nagata Y, et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci U S A 2000;97:12198–12203.
Odunsi K, Qian F, Matsuzaki J, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A 2007;104:12837–12842.
Caballero OL, Chen YT. . Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci 2009;100:2014–2021.
Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29:917–924.
De Smet C, Lurquin C, Lethé B, et al. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol 1999;19:7327–7335.
Woloszynska-Read A, Mhawech-Fauceglia P, Yu J, et al. Intertumor and intratumor NY-ESO-1 expression heterogeneity is associated with promoter-specific and global DNA methylation status in ovarian cancer. Clin Cancer Res 2008;14:3283–3290.
Acknowledgements
Jessica Gillespie performed the quantitative real-time PCR and western analyses of CTAG1B and NY-ESO-1 expression.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hemminger, J., Ewart Toland, A., Scharschmidt, T. et al. The cancer-testis antigen NY-ESO-1 is highly expressed in myxoid and round cell subset of liposarcomas. Mod Pathol 26, 282–288 (2013). https://doi.org/10.1038/modpathol.2012.133
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.133
Keywords
This article is cited by
-
Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy
Journal of Translational Medicine (2020)
-
Establishment and characterization of a new human myxoid liposarcoma cell line (DL-221) with the FUS-DDIT3 translocation
Laboratory Investigation (2016)
-
NY-ESO-1 (CTAG1B) expression in mesenchymal tumors
Modern Pathology (2015)
-
Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma
Modern Pathology (2014)
-
Systemic treatment of soft-tissue sarcoma—gold standard and novel therapies
Nature Reviews Clinical Oncology (2014)