Abstract
We have reported previously that duodenal follicular lymphoma (FL) is distinct from nodal FL and showed more resemblance to mucosa-associated lymphoid tissue lymphoma, and that FL frequently involved the duodenal second portion. In the present study, we examined duodenal FLs and gastric/colonic FLs to clarify the clinicopathological and immunological differences between the tumor types. We analyzed 8 samples of gastric FL, 17 of duodenal ones, and 5 of colonic/rectal ones, and characterized them by immunohistochemistry, immunogenotyping, and histology. Gastric and colonic FLs presented in submucosal to subserosal areas, whereas duodenal ones presented in the mucosal to submucosal layers. Immunohistochemical analysis revealed that duodenal FLs exhibited the following phenotypes: CD10 (+), B-cell lymphoma 2 (BCL-2) (+), BCL-6 (+), activation-induced cytidine deaminase (AID) (−), BACH2 (+), CD27 (+), MUM-1 (−), Blimp-1 (−), and loose CD21 network (duodenal pattern). Gastric/colonic FLs exhibited the following phenotypes: CD10 (+), BCL-2 (+), BCL-6 (+), AID (+), BACH2 (+), CD27 (−), MUM-1 (−), Blimp-1 (−), and a dense CD21 network (nodal pattern). Expression of AID and CD27 in lymphoma cells and the CD21 network pattern were considerably different between duodenal FLs and gastric/colonic ones. Moreover, in situ hybridization revealed that, in the duodenal FLs, BACH2 was expressed at the periphery of the tumor follicle and tumor villi. The number of immunoglobulin heavy-chain variable domains VH4 and VH5 were higher in duodenal follicular lymphomoas than in gastric FLs. The lymphoma cells of duodenal FLs are different from those of gastric/colonic FLs, and duodenal FL is distinct even within the gastrointestinal tract. Somatic hypermutation in immunoglobulin genes and CD27 expression are hallmarks of memory B cells. We suggest that duodenal FL cells are in the memory B-cell stage, and require BACH2 instead of AID for ongoing mutation.
Similar content being viewed by others
Main
We have reported that duodenal follicular lymphoma (FL) is a distinct FL by virtue of lacking follicular dendritic cells (FDC), activation-induced cytidine deaminase (AID), and immunoglobulin variable heavy-chain deviation.1, 2 On the other hand, we have also reported that duodenal FLs harbor t(14;18)(IGH-BCL-2), exhibit ongoing somatic hypermutations, and express CD10 and B-cell lymphoma 2 (BCL-2), which are common features of nodal FL. However, the proteins have roles in somatic hypermutation in duodenal FL have not yet been identified.
Although the duodenal second portion is the most frequent site of FL in the gastrointestinal tract,3, 4 it sometimes occurs primarily in the gastrointestinal tract outside of the duodenum. Using double-balloon and/or capsule endoscopy, FLs of the gastrointestinal tract primarily involve the duodenum and frequently spread to the small intestine,3 Few FLs occur in the stomach and colon, but the clinicopathological features of FL in such organs remain unclear. AID has a key role in class switching and somatic hypermutation in B cells,5 and BACH2 also has a role in these processes in B cells.6 BACH2 and Bcl-6 suppress Blimp-1, which is a key regulator of plasma-cell differentiation.7 We have previously reported the lack of AID expression in duodenal FLs, but the entity associated with somatic hypermutation and ongoing mutation remains unknown. Therefore, we focused on BACH2 expression, especially in duodenal FLs, and sought to clarify the differences between duodenal and other gastrointestinal FLs with special reference to the characteristics of FDC, the expression of AID and BACH2, and stage of differentiation in lymphoma cells.
Materials and methods
Patients
Subjects included 8 patients with FL in the stomach, 5 with FL in the colon and rectum, and 17 with FL in the duodenum, which were previously reported.1 We obtained three samples of reactive lymphoid hyperplasia of the lymph node and three of the duodenum for CD27 immunohistochemical control specimens. Informed consent to use the samples was obtained from all the patients.
Immunohistochemical Analysis
Immunohistochemical staining was performed on sections from 10% buffered formalin-fixed and paraffin-embedded tissues using heat-induced epitope retrieval or trypsin-induced retrieval, an avidin–biotin complex method, and an automated immunostainer (Ventana Medical System, Tuscon, AZ, USA), as previously described.8 The antibody panel used to assess these cases was as follows (clone, dilution): CD20 (L26, 1:200), CD3 (PS-1, 1:50), CD10 (56C6, 1:50), CD5(4C7, 1:100), Bcl-2 (3.1, 1:200), CD23 (1B12, 1:100), CD27 (137B4, 1:50), and Ki-67 (MIB-1, 1:5000) (Novocastra, Newcastle-upon-Tyne, UK); CD21 (1F8, 1:20), MUM-1 (MUM1p, 1:50) (DAKO Cytomation, Denmark); Bcl-6 (D-8, 1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); cyclin D1 (SP4, ready to use) (Nichirei, Japan); AID (ZA001, 1:100) (Zymed, South San Francisco, CA, USA); and Blimp-1 (3H2-E8, 1:200) (Novus Biologicals, Littleton, CO, USA). Rabbit polyclonal anti-human BACH2 antibody (F69-2) was used as a primary antibody at a dilution of 1:500. Muto et al6 recently reported that staining with the anti-BACH2 antibody was severely diminished in the spleens of BACH2-deficient mice, verifying the specificity of the antibody. For CD20, CD3, CD10, CD5, Cyclin D1, Bcl-2, Bcl-6, and MUM-1 antigens, samples were scored as positive when 30% or more of lymphoma cells were positively stained. For AID expression in tumor follicles, samples with 5% or more expressing cells were scored as positive as previously described.9 Ki-67-positive cells were counted in tumor follicles. CD21 expression patterns were classified as follows: nodal (>30% positive cells); intermediate (5–30% positive cells); and duodenal (<5% positive cells and FDC located at the periphery of tumor follicles).
Fluorescence In Situ Hybridization (FISH)
FISH for t(14;18)(q32;q21)/IGH-BCL-2 translocations was performed using the LSI BCL-2 FISH DNA fusion signal probe (Abbott Molecular, Wiesbaden, Germany) according to the manufacturer’s instructions. We performed FISH directly on paraffin-embedded tissue sections and detected the hybridization signal as previously described.10
In Situ Hybridization
In situ hybridization was performed on paraffin-embedded tissue sections using a BACH2 RNA probe, which was designed using a modified multi-labeling method as previously described.11
DNA Extraction and PCR
DNA was extracted from paraffin-embedded tissue using the QIAmp DNA Micro Kit (Qiagen, Valencia, CA, USA). The variable regions (CDR2 and FW3) and VDJ region (CDR3) of the immunoglobulin heavy-chain (IgVH) gene were amplified by semi-nested PCR, using the primers of FR2, LJH, and VLJH as described earlier.1, 12 Primers used were as follows: 5′-CCGGRAARRGTCTGGAGTGG-3′, as upstream consensus V region primer (FR2); 5′-CTTACCTGAGGAGACGGTGACC-3′, as a consensus J region primer (LJH); 5′-GTGACCAGGTNCCTTGGCCCC-3′, as a consensus J region primer (VLJH). PCR products were purified using the QIAquick PCR purification kit (Qiagen). Then, 1 μl of the PCR product was used for direct sequencing (ABI PRISM Model 3100, version 3.7, Applied Biosystems). Next, the resulting immunoglobulin sequence was fed into BLAST (NCBI) to identify the closest germline sequences.
Western Blotting
CD27 protein expression in tumor samples was determined by western blot analysis. Protein lysates were analyzed using standard techniques and the anti-CD27 rabbit polyclonal antibody (Abcam, Tokyo, Japan) as described.13 We included one fresh-frozen reactive lymphoid hyperplasia sample as a positive control, a HeLa cell line sample as a negative control, and three fresh-frozen duodenal FL samples in each analysis.
Statistical Analysis
All statistical analyses were performed with the Mann–Whitney U-test using SPSS software (version 14.0; SPSS, Chicago, IL, USA). Values of P<0.05 were considered statistically significant.
Results
Clinicopathological Findings
Clinical features (age, gender, and clinical stage), histological grading, and immunohistochemical findings are summarized in Table 1. The study group comprised 16 men and 14 women, aged between 40 and 81 years, with a median age of 61 years (Stomach FL: age range, 51–81 years; median age, 63 years. Duodenum FL: age range, 49–75 years; median age, 61 years. Colon and rectum FL: age range, 40–87 years, median age, 58 years). Clinical stages were determined according to the criteria recommended by the International Workshop (Lugano) and are detailed in Table 1. All stage IV patients had bone marrow involvement. We excluded patients with multiple lymph node lesions. In our patient series, the stage IV patients had both gastrointestinal lesions and bone marrow lesions, and they had predominant gastrointestinal involvement. Typical histological appearance of patient samples showed a vague nodular pattern composed of small- to medium-sized cleaved lymphoid cells. The main locations of gastric and colonic FLs were the submucosal to subserosal areas (Figures 1a–f). Duodenal FLs were located in the submucosal area (Figures 1g–i), and tumor cells were associated with duodenal villi. Histological grades were distributed as follows: grade 1: 24 samples; grade 2: 5 samples; Grade 3A: 1 sample.
Pathological features of gastric, duodenal, and colonic/rectal follicular lymphoma (FL). (a) Gastric FL (HE stain; low power field). Tumor cells are present in the proper muscle to subserosal area. (b) CD10 immunostaining of gastric FL (low power field). (c) B-cell lymphoma 2 (BCL-2) immunostaining of gastric FL (low power field). (d) Gastric FL (HE. stain). Vague nodular tumor follicles are present. (e) CD10 immunostaining of gastric FL. Tumor cells are positive. (f) BCL-2 immunostaining of gastric FL. Tumor cells are positive. (g) Colonic FL (HE stain). Tumor cells are present in the musculature proper area. (h) CD10 immunostaining of colonic FL. Tumor cells are positive. (i) BCL-2 immunostaining of colonic FL. Tumor cells are positive. (j) Duodenal FL (HE stain). Tumor cells are present in the lamina propria area. (k) CD10 immunostaining of duodenal FL. Tumor cells are positive. (l) BCL-2 immunostaining of duodenal FL. Tumor cells are positive.
Immunophenotyping Results
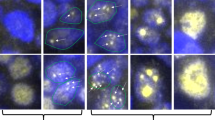
CD21 expression patterns are shown in Table 2. FDC were arranged at the periphery of tumor follicles in 15 of 17 duodenal FLs as previously described (Figure 2a.1 In contrast, among the eight stomach FLs, seven exhibited a nodal pattern and one showed an intermediate pattern (Figure 2b). Out of five patient samples in the colon and rectum, three were nodal and two were intermediate (Figures 2c and d). Statistical comparison of the expression patterns of duodenal FLs and gastric/colonic FLs showed that the distributions were significantly different (P<0.001).
CD21 and AID immunohistochemistry in duodenal, gastric, and colonic FLs. (a) CD21 immunostaining of duodenal FL. Follicular dendritic cells (FDC) present at the periphery of tumor follicles (duodenal pattern). (b) CD21 immunostaining of gastric FL. FDC networks densely present in tumor follicles (nodal pattern). (c) CD21 immunostaining of colonic FL. FDC networks densely present in tumor follicles (nodal pattern). (b) Intermediate pattern of CD21 immunostaining in colonic FL. (e) AID immunostaining in duodenal FL. Tumor cells are negative. (f) AID immunostaining of gastric FL. Tumor cells are positive. (g) BACH2 immunostaining in duodenal FL. Periphery of tumor follicles and villi are positive, but center of tumor follicle is negative. (h) BACH2 immunostaining of gastric FL. Tumor follicles are positive.
Results of AID and BACH2 expression analyses are also shown in Table 2. Only 1 of 17 duodenal FLs expressed AID (Figure 2e), whereas 6 of 8 gastric FLs were positive for AID (Figure 2f). All colonic and rectal samples were positive for AID. BACH2 expression was comparatively different: 14 of 17 duodenal FL samples were positive for BACH2. Interestingly, the AID-positive sample (no. 20) was negative for BACH2. In seven samples, BACH2 was expressed at the periphery of the tumor follicle and tumor villi (Figure 2g, Table 2; shown as (+)). BACH2 was also commonly expressed in other FLs: six of eight gastric FLs were positive for BACH2 (Figure 2h) as were three of five colonic/rectal FLs.
In situ hybridization analysis of BACH2 expression confirmed the immunohistochemical findings. Tumor cells at the peripheral zone of tumor follicles and at the villi expressed BACH2 mRNA (Figures 3a and b). This pattern corresponded with the protein expression pattern. We also examined Blimp-1 expression and found no Blimp-1 expression in any of the patient samples (Table 2).
BACH2 and CD27 expression. (a) BACH2 in situ hybridization in duodenal follicular lymphoma (FL) sample. Levels of mRNA expression correlate with protein expression. Inset of Figure 3a shows detection of BACH2 mRNA with sense DIG-labeled probes as a negative control. (b) Same sample as Figure 3a. Tumor at the villi also expresses BACH2 at the mRNA level. (c) CD27 expression in reactive lymphoid hyperplasia of the duodenal sample. Positive cells (active B cells and plasma cells) are present at the villi. (d) CD27 immunostaining of reactive lymphoid hyperplasia in the tonsil sample. Positive cells are scattered in the germinal center light zone and interfollicular zone. (e) CD27 expression in duodenal FL. Tumor cells are strongly positive. (f) CD27 expression in colonic FL. Tumor follicles are negative.
We examined differentiation in lymphoma cells using CD27 staining. As both mutated IgM+ B-cell populations and the class-switched memory B cells share expression of the TNF-receptor superfamily member CD27, which is not expressed in unmutated naive B cells,14, 15 CD27 is thought to be a memory B-cell marker.16 Figure 3c shows CD27 expression in the reactive lymphoid hyperplasia of the duodenum: the CD27-positive B cells or plasma cells were present at the villi and lymphoid follicle. CD27 expression is also shown in the reactive hyperplastic germinal centers of the tonsil: the positive cells were scattered in the germinal center (prominent in light zone) and interfollicular zone (Figure 3d).
As shown in Table 2, 15 of 17 duodenal FL samples strongly expressed CD27 (Figure 3e). Western blotting analysis also confirmed CD27 protein expression in duodenal FL (Supplementary Figure 1). In eight gastric and seven colonic FL samples, all samples were negative for CD27 (Figure 3f), except those of patients no. 7 and no. 26. Statistical comparison of expression patterns in duodenal FLs and nodal, gastric, and colon FLs showed that the distributions are significantly different (P<0.001).
IgVH Gene Usage
We could detect monoclonal bands in six of eight gastric FL samples and one of five colonic/rectal FL samples. These results are shown in Table 2. In gastric FL, five of six were VH3 and one was VH4. In duodenal FL, 9 of 17 were VH3, 5 were VH4, and 3 were VH5.
Fluorescence In Situ Hybridization
The t(14;18) translocation was detected in 13 of 15 (about 87%) duodenal FL samples, 3 of 8 (38%) gastric FLs, and 3 of 5 (60%) colon and rectal FLs (Figure 4a). In gastric FL samples, the frequency of the translocation was significantly lower than that in duodenal FLs (P=0.015). Furthermore, gastric FLs with lower clinical stages (Stage I–II1, localized tumor stage) had a lower tendency to exhibit the t(14;18) translocation (Table 2). The IGH-BCL-2 translocation was more frequent in duodenal samples (87%) than in gastric (40%) and colonic (59%) FLs (P=0.008).
Discussion
Although the gastrointestinal FLs are relatively rare, accounting for 1–3.6% of all gastrointestinal lymphomas, duodenal FLs have recently been found with increasing frequency by upper gastrointestinal endoscopic examination. These FLs are characterized by lower clinical stage, lower histological grading, and better prognosis. Gine et al17 reported that the frequency of clinical stage III–IV in nodal FL was 81%. Solal-Celigny et al18 found a similar frequency of 78%. In our previous multicenter retrospective study, 41 of 191 patients with gastrointestinal FL (21.5%) were stage IV.4 Histological differences between duodenal FLs and stomach and colon FLs involved tumor depth, size of individual tumor follicles and the size of the lesions. Duodenal FLs were mainly located in submucosal areas, and tumor cells were present in mucosal villi. Macroscopically, duodenal FLs typically present as small white multiple nodules. These histological differences suggest the association of a mucosal homing receptor such as α4β7 as reported by Bende et al.19 Most duodenal lymphoma samples are obtained by biopsy, and the assessment thus performed is considered insufficient. However, in our previous patient series, a few patients were examined by ultrasonic endoscopy. All patients who had macroscopically multiple white nodules had a limited submucosal layer. In our present series of duodenal FLs, all macroscopic types were found to be typical multiple white nodules. The patients in the present series were examined by CT and/or MRI. We think we should examine further studies in the future.
Both normal germinal centers and nodal FLs have dense CD21-positive follicular dendritic cell networks.20 We previously showed that duodenal FL lacked follicular dendritic cell networks, with dendritic cells distributed at the periphery of the tumor follicles.1 In contrast, other gastrointestinal tract (stomach, colon, and rectum) FLs had dense follicular dendritic cell networks similar to those of nodal FLs. The follicular dendritic cell network pattern may depend on the nature of the primary tumor. For example, we documented a patient with primary FL in the inguinal lymph node, who achieved complete remission by chemotherapy and then relapsed with lesions at duodenal and gastric sites. Interestingly, the nodal and two gastrointestinal sites showed the same follicular dendritic cell pattern (nodal pattern, data not shown). Conversely, some duodenal and nodal samples from patients presenting with systemic lymphadenopathy showed the same dendritic cell pattern (duodenal pattern, data not shown).
An important question concerns whether tumor cells invading follicles arise from the villi or whether they spread from tumor follicles to the villi. Figure 4b presents a hypothetical schema of development of duodenal FL. The VH-usage deviation and memory-cell characters strongly suggested that the presence of antigen stimulation, chemokines, and adhesion molecules probably affect tumor spreading. One hypothesis is that tumor cells originating in the villi spread to other villi and invade non-tumor follicles. Tumor cells invading non-tumor follicles disrupt the follicular dendritic cell network, as seen in follicular colonization in MALT lymphoma. Another hypothesis is that lymphoma cells from tumor follicles spread to the villi.
AID has a key role in class switching and somatic hypermutation in B cells.5 BACH2 also has a role in these processes in B cells.6 BACH2 and Bcl-6 suppress Blimp-1, which is a key regulator of plasma-cell differentiation.7 We have previously reported a lack of AID expression in duodenal FLs. In the present study, 14 of 17 samples of duodenal FL expressed BACH2 protein and mRNA. In seven samples, the BACH2 pattern and the pattern of tumor cells in the villi and periphery of the tumor follicles was the same. This unique pattern was not found in nodal, gastric, or colonic FLs. This difference was quite interesting, but the mechanism by which AID and BACH2 are differentially expressed remains unclear. We cannot clearly demonstrate this association in our present study, and further studies will be required to clarify this association.
Most duodenal follicular samples expressed BACH2, and all lacked Blimp-1, which is repressed by BACH2 and Bcl-6. Thus, BACH2 might have a key role in ongoing somatic hypermutation in duodenal FLs. Sakane-Ishikawa et al21 described better prognostic value of BACH2 expression in diffuse large B-cell lymphomas. Takakuwa et al22 described an inhibitory effect of BACH2 on proliferation of Raji cell lines. In previous reports, BACH2 was reported to have a tumor suppressor role in B-cell lymphomas. On the other hand, AID is associated with lymphomagenesis and AID expression is related to poor prognosis in several B-cell lymphoma subtypes.9, 23, 24 We do not have sufficient data for long-term follow-up of patients with duodenal FL, but based on these reports, we suggest that BACH2 expression and lack of AID expression might limit the tumor stage and lead to better prognosis in duodenal FL.
Expression of CD27 was common (15 of 17) in the duodenal FL samples. In 16 Grade 1 nodal FLs, all patient samples were negative for CD27 (data not shown). In five gastric MALT lymphoma patient samples, four samples were positive and one was negative for CD27 (data not shown). In B-cell differentiation, postgerminal center B cells, selected by affinity maturation and induced by somatic hypermutation, differentiate to plasmablasts or memory B cells.25 CD27 is a tumor necrosis factor receptor superfamily member and a general human memory B-cell marker. In human normal intestinal mucosa, scattered secretory IgA+ CD27+ memory B cells are present in the lamina propria, but are scarce in gut-associated lymphoid tissue.26 In duodenal FL samples, tumor cells expressed IgA, CD27, and BACH2. Thus, tumor cells differentiate to memory B cells with somatic and ongoing hypermutations. For this reason, we note that these data suggest a resemblance of duodenal FL to MALT lymphoma. CD27+ memory B cells are subclassified as IgM+IgD+, IgM+IgD-, IgG+IgA+, and IgM-IgD+.27 Thus, duodenal FLs are subclassified as IgA+ memory B-cell like. Dong HY et al28 described that CD27 did not distinguish between neoplastic B cells of naive versus memory type. Furthermore, Schmitter D et al29 described CD27 expression in CD70-stimulated FL cells. Somatic hypermutation by itself is a feature of both follicular center and memory B cells, and CD27 is also expressed by some follicular center cells. Therefore, we should be more conservative in the precise maturational state of the duodenal FL cells. However, there might also be difference between nodal FL and duodenal FL in terms of CD27 expression.
In gastric and colonic FLs, VH3 gene usage was frequently observed, but in our samples, we could detect too few monoclonal bands to allow comparison with duodenal samples. More samples will be required to compare VH gene usage. Translocations of t(14;18)(q32;q21) and IGH-BCL-2 are considered to be hallmarks of FL,29, 30 but t(14;18) is present in about half the healthy samples.31, 32 In gastric FL of lower clinical stage, there were no IGH/BCL-2 translocations. These facts suggest that gastric FL may be similar to primary cutaneous follicle center lymphoma.33,34
In conclusion, we suggest that duodenal FL is a distinct entity among gastrointestinal FLs, by virtue of AID loss but BACH2 expression, displaying a specific CD21 pattern, and high frequency of CD27 expression.
References
Takata K, Sato Y, Nakamura N et al Duodenal and nodal follicular lymphomas are distinct: the former lacks activation-induced cytidine deaminase and follicular dendritic cells despite ongoing somatic hypermutations. Mod Pathol 2009;22:940–949.
Sato Y, Ichimura K, Tanaka T et al Duodenal follicular lymphomas share common characteristics with mucosa-associated lymphoid tissue lymphomas. J Clin Pathol 2008;61:377–381.
Kodama M, Kitadai Y, Shishido T et al Primary follicular lymphoma of the gastrointestinal tract: a retrospective case series. Endoscopy 2008;40:343–346.
Takata K, Okada H, Ohmiya N et al Primary gastrointestinal follicular lymphoma involving the duodenal second portion is a distinct entity: A multicenter, retrospective analysis in Japan. Cancer Sci 2011;102:1532–1536.
Muramatsu M, Kinoshita K, Fagarasan S et al Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000;102:553–563.
Muto A, Tashiro S, Nakajima O et al The transcriptional programme of antibody class switching involves the repressor BACH2. Nature 2004;429:566–571.
Igarashi K, Ochiai K, Muto A . Architecture and dynamics of the transcription factor network that regulates B-to-plasma cell differentiation. J Biochem 2007;141:783–789.
Khoury JD, Jones D, Yared MA et al Bone marrow involvement in patients with nodular lymphocyte predominant Hodgkin lymphoma. Am J Surg Pathol 2004;28:489–495.
Leuenberger M, Frigerio S, Wild PJ et al AID protein expression in chronic lymphocytic leukemia/small lymphocytic lymphoma is associated with poor prognosis and complex genetic alterations. Mod Pathol 2010;23:177–186.
Takada S, Yoshino T, Taniwaki M et al Involvement of the chromosomal translocation t(11;18) in some mucosa-associated lymphoid tissue lymphomas and diffuse large B cell lymphomas of the ocular adnexa: evidence from multiplex reverse transcriptase –polymerase chain reaction and fluorescence in situ hybridization on using formalin-fixed, paraffin embedded specimens. Mod Pathol 2003;16:445–452.
Mori H, Nomura T, Seno M et al Expression of phospholipid hydroperoxide glutathione peroxidase (PHGPx) mRNA in rat testes. Acta Histochem Cytochem 2001;34:25–30.
Nakamura N, Kuze T, Hashimoto Y et al Analysis of the immunoglobulin heavy chain gene variable region of CD5-positive and –negative diffuse large B cell lymphoma. Leukemia 2001;15:452–457.
Gajjar A, Hernan R, Kocak M et al Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol 2004;22:984–993.
Agematsu K, Nagumo FC, Yang FC et al B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur J Immunol 1997;27:2073–2079.
Klein U, Rajewsky K, Kuppers R . Human immunoglobulin IgM+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 1998;188:1679–1689.
Seifert M, Kuppers R . Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med 2009;16:2659–2669.
Gine E, Montoto S, Bosch F et al The follicular lymphoma international prognostic index (FLIPI) and the histological subtype are the most important factors to predict histological transformation in follicular lymphoma. Ann Oncol 2006;17:1539–1545.
Solal-Celigny P, Roy P, Colombat P et al Follicular lymphoma international prognostic index. Blood 2004;1:1258–1265.
Bende RJ, Smit LA, Bossenbroek JG et al Primary follicular lymphoma of the small intestine: alpha4beta7 expression and immunoglobulin configuration suggest an origin from local antigen-experienced B cells. Am J Pathol 2003;162:105–113.
Kagami Y, Jung J, Choi YS et al Establishment of a follicular lymphoma cell line (FLK-1) dependent on follicular dendritic cell line HK. Leukemia 2001;15:148–156.
Sakane-Ishikawa E, Nakatsuka S, Tomita Y et al Prognostic significance of BACH2 expression in diffuse large B-cell lymphoma: A study of the Osaka lymphoma study group. J Clin Oncol 2005;23:8012–8017.
Takakuwa T, Luo WJ, Ham MF et al Integration of Epstein-Barr virus into chromosome 6q15 of Burkitt lymphoma cell line (Raji) induces loss of BACH2 expression. Am J Pathol 2004;164:967–974.
Gu X, Shivarov V, Strout MP . The role of activation-induced cytidine deaminase in lymphomagenesis. Curr Opin Hematol 2012;19:292–298.
Dijkman R, Tensen CP, Buettner M et al Primary cutaneous follicle center lymphoma and primary cutaneous large B-cell lymphoma, leg type, are both targeted by aberrant somatic hypermutation but demonstrate differential expression of AID. Blood 2006;107:4926–4929.
Allen CDC, Okada T, Cyster JG . Germinal-center organization and cellular dynamics. Immunity 2007;27:190–202.
Brandtzaeg P, Johansen FE . Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev 2005;206:32–63.
Berkowska MA, Driessen GJA, Bikos V et al Human memory B cells originate from three distinct germinal center-dependent and –independent maturation pathways. Blood 2011;118:2150–2158.
Dong HY, Shahasafaei A, Dorfman DM . CD148 and CD27 are expressed in B cell lymphomas derived from both memory and naïve B cells. Leuk Lymphoma 2002;43:1855–1858.
Schmitter D, Bolliger U, Hallek M et al Involvement of the CD27-CD70 co-stimulatory pathway in allogenic T-cell response to follicular lymphoma cells. Br J Haematol 1999;106:64–70.
Helen MC, David BJ, Dennis HW . Cytogenetic and molecular studies of t(14;18) and t(14;19) in nodal and extranodal B-cell lymphoma. J Pathol 1992;166:129–137.
Albinger-Hegyi A, Hochreutener B, Abdou MT et al High frequency of t(14;18)-translocation breakpoints outside of major breakpoints and minor cluster regions in follicular lymphomas. Am J Pathol 2002;160:823–832.
Hirt C, Dölken G, Janz S et al Distribution of t(14;18)-positive, putative lymphoma precursor cells among B-cell subsets in healthy individuals. Br J Haematol 2007;138:349–353.
Roulland S, Navarro JM, Grenot P et al Follicular lymphoma-like B cells in healthy individuals: a novel intermediate step in early lymphomagenesis. J Exp Med 2006;203:2425–2431.
Streubel B, Scheucher B, Valencak J et al Molecular cytogenetic evidence of t(14;18)(IGH;BCL2) in a substantial proportion of primary cutaneous follicle center lymphomas. Am J Surg Pathol 2006;30:529–536.
Acknowledgements
This work was supported in part by grants from the Japan Society for the Promotion Science (JSPS no. 19590348) and was supported in part by a Grant-in-Aid for Cancer Research (21-6-3) from the Ministry of Health, Labor and Welfare, Tokyo, Japan. We special thanks Ms H Nakamura, Ms M Okabe, Dr T Kunitomo, Dr A Uchiyama, Dr S Nose and Dr T Miyake for their technical assistance and preparing pathological samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Takata, K., Sato, Y., Nakamura, N. et al. Duodenal follicular lymphoma lacks AID but expresses BACH2 and has memory B-cell characteristics. Mod Pathol 26, 22–31 (2013). https://doi.org/10.1038/modpathol.2012.127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.127
Keywords
This article is cited by
-
Density and size of lymphoid follicles are useful clues in differentiating primary intestinal follicular lymphoma from intestinal reactive lymphoid hyperplasia
Diagnostic Pathology (2020)
-
Update on lymphoproliferative disorders of the gastrointestinal tract: disease spectrum from indolent lymphoproliferations to aggressive lymphomas
Virchows Archiv (2020)
-
Elevation of serum interleukins 8, 4 and 1β levels in patients with gastrointestinal low-grade B-cell lymphoma
Scientific Reports (2015)