Abstract
Glycine N-methyltransferase (GNMT) has a role in the metabolism of methionine as well as in gluconeogenesis. It has recently been reported that the GNMT gene acts as a tumor-susceptible gene. However, little is known about the specific function of GNMT in carcinogenesis and malignant progression. To better our understanding of the function of GNMT in prostate cancer, we used siRNAs to examine the effects of GNMT knockdown on cell proliferation and the cell cycle. In addition, the relation between immunohistochemical GNMT expression and clinicopathologic parameters was investigated in 148 prostate cancer tissues. Here, we show that siRNA-mediated GNMT knockdown results in an inhibition of proliferation, and induces G1 arrest and apoptosis in prostate cancer cell lines. Moreover, high cytoplasmic GNMT expression was also correlated with a higher Gleason score (P<0.001) and higher pT stage (P=0.027). The patients with high GNMT cytoplasmic expression showed significantly lower disease-free survival rates than patients with low expression (P<0.001). High GNMT cytoplasmic expression had a significant impact on patient disease-free survival in multivariate analysis (P=0.005). This is the first investigation to reveal the novel finding that GNMT may have an important role in promoting prostate cancer cell growth via the regulation of apoptosis and contribute to the progression of prostate cancer. The modulation of GNMT expression or function may be a strategy for developing novel therapeutics for prostate cancer. GNMT may represent a novel marker of malignant progression and poor prognosis in prostate cancer.
Similar content being viewed by others
Main
Prostate cancer is one of the most common solid cancers.1, 2 Most men with this tumor have a somewhat better prognosis, and it is becoming clear that some men may even not benefit from either the diagnosis or treatment of prostate cancer. In patients with localized prostate cancer, operative therapy is effective; however, a substantial number of patients will experience recurrent disease.3 Recurrence in patients with prostate cancer is still difficult to predict, and the emergence of effective new approaches for therapy will depend on clarification of the mechanisms involved in the progression of this cancer.
Although gene and protein expression have been extensively profiled in prostate cancer, little is known about the global metabolomic alterations that are involved in the progression. Metabolomics is particularly valuable for cancer research because individual metabolites represent end points of the molecular pathways that are perturbed by other factors such as the genome, transcriptome, and proteome.4 Sreekumar et al have profiled >1126 metabolites across 262 clinical samples related to prostate cancer. Sarcosine, an N-methyl derivative of the amino-acid glycine, was identified as a differential metabolite that was highly increased during prostate cancer progression to metastasis and could be detected in urine.5 Sarcosine levels were also found to be increased in invasive prostate cancer cell lines relative to benign prostate epithelial cells.5
Glycine N-methyltransferase (GNMT) is a 295-amino-acid protein that catalyzes the methylation of glycine by using S-adenosylmethionine to form sarcosine (N-methylglycine) with the concomitant production of S-adenosylhomocysteine. GNMT was identified as a cytosolic protein, and the enzyme has been suggested to have an important role in the metabolism of methionine in the liver.6, 7 It has been postulated that GNMT is involved in gluconeogenesis in the liver and kidney.8
Knockdown of GNMT in the prostate cancer cell line by RNA interference attenuated prostate cancer invasion in the cell invasion assay.5 Their data are suggestive of a link between cancer progression and GNMT activity.
By contrast, the expression of GNMT has been observed to be downregulated in a human hepatocellular carcinoma cell line and tissue.9 A loss of heterozygosity at the GNMT in pairs of normal and tumor tissue from HCC patients has also been observed,10 and GNMT knockout mice develop liver cancer.11 A significant proportion of human prostate cancers show a loss of heterozygosity of the GNMT gene.12 Furthermore, immunohistochemical results show abundant GNMT expression in normal tissues, whereas this expression is diminished in prostate cancer tissues12 and cholangiocarcinoma tissues.13 These studies suggest that GNMT is a tumor susceptibility gene for prostate cancer. GNMT had different roles in carcinogenesis and progression between the studies of Sreekumar et al5 and those of others.10, 12, 13 It therefore remains controversial whether the GNMT gene is tumor susceptible.
It is therefore necessary to determine the significance of GNMT in prostate cancer carcinogenesis and progression. In this study, we used siRNAs to examine the effects of GNMT knockdown on cell proliferation and the cell cycle. Furthermore, the relationship between immunohistochemical GNMT expression and clinicopathologic parameters was investigated in prostate cancer tissues.
Materials and methods
Cell Culture
Human normal prostate epithelial RWPE-1 (keratinocyte serum-free medium), human prostate cancer PC-3 (Eagle's Minimal Essential Medium, MEM), DU145 (Dulbecco's Modified Eagle's Medium, DMEM), VCaP (DMEM), 22Rv1 (RPMI1640), and LNCaP cells (RPMI1640) were cultured in the media as indicated in the parentheses. These media were purchased from Invitrogen (San Diego, CA, USA) and contained 10% fetal bovine serum. The cell lines were maintained in a 5% CO2 atmosphere at 37°C.
Antibodies
Anti-GNMT (sc-68871, rabbit polyclonal) and anti-PARP (sc-1561, goat polyclonal) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-cleaved-PARP (anti-PARP p85 fragment pAb, G7341, rabbit polyclonal) and anti-β-actin (A5441, mouse monoclonal) antibodies were purchased from Promega (Madison, WI, USA) and Sigma (St Louis, MO, USA), respectively.
Western Blotting
Whole-cell extracts were prepared as previously described.14, 15, 16 The protein concentrations were determined using a Protein Assay Kit (Bio-Rad, Hercules, CA, USA) based on Bradford's method. Whole-cell extracts (30 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidine difluoride microporous membranes (Micron Separations, Westborough, MA, USA) using a semi-dry blotter. The blotted membranes were incubated for 1 h at room temperature with a primary antibody. Membranes were then incubated for 40 min at room temperature with a peroxidase-conjugated secondary antibody. The bound antibody was visualized using an ECL kit (GE Healthcare Bio-Science, Piscataway, NJ, USA), and membranes were exposed to Kodak X-OMAT film.
Knockdown Analysis Using siRNAs
Knockdown analysis using siRNA was performed as described previously.14, 15, 16 The following double-stranded RNA 25-basepair oligonucleotides were commercially generated (Invitrogen): 5′-AAGUGAGCGAAACUGUUUCCAAGGC-3′ (sense) and 5′-GCCUUGGAAACAGUUUCGCUCACUU-3′ (antisense) for GNMT siRNA #1; 5′-AUGUUGUGACGUCCUUGGUCAAGUC-3′ (sense) and 5′-GACUUGACCAAGGACGUCACAACAU-3′ (antisense) for GNMT siRNA #2. RWPE-1, PC-3, and LNCaP cells were transfected with the indicated amounts of siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Cell Proliferation Assay
The cell proliferation assay was performed as described previously.14, 15 RWPE-1, PC-3, and LNCaP cells (2.5 × 104) were seeded in 12-well plates and transfected with 40 nmol/l of siRNA, as described above. Twelve-hour post-transfection was set as 0 h. The cells were harvested with trypsin and counted daily using a cell counter (Beckman Coulter, Fullerton, CA, USA). The results were normalized to cell counts at 0 h, and are representative of at least three independent experiments.
Flow Cytometry
Knockdown analysis using siRNA was performed as described previously.14 RWPE-1, PC-3, and LNCaP cells (2.5 × 105) were seeded in six-well plates, transfected with the 40 nmol/l of siRNA, and cultured for 72 h. The cells were harvested, washed twice with ice-cold phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA), and resuspended in 70% ethanol. After being washed twice with ice-cold PBS, cells were resuspended in PBS containing 0.1% BSA, incubated with RNase, and stained with propidium iodide (Sigma). Cells were analyzed using a FACS Calibur (BD Biosciences, San Jose, CA, USA).
Tissues and Clinical Data
The subjects were 148 patients who received radical prostatectomy with no chemotherapy or hormonal therapy before the operation at the Kyushu University hospital, Japan, between 1997 and 2006 and who had enough cancer area for the evaluation of immunohistochemistry (Table 1). All patients underwent surgery for clinically localized prostate cancer as determined by pre-operative prostate-specific antigen (PSA) concentrations, digital rectal examination, and prostate needle biopsy. All prostatectomy specimens were completely reviewed to establish the stage and grade of the respective prostate cancers. Slides for this study were prepared from the prostate blocks that contained the largest and representative area of the tumor and adjacent normal epithelium. We removed identifying information from all samples for privacy protection. This study was conducted in accordance with the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese Government. This study was conducted in accordance with the institutional review board approval.
Four patients received hormonal therapy after surgery. The follow-up period for 16 patients was <2 years. We excluded these 20 patients from disease-free survival analysis. Follow-up data were available for 128 patients. They were monitored for serum PSA. A PSA level >0.4 ng/ml was defined as PSA recurrence. Recurrence was found in 20% (26/128) of patients. The median follow-up period of patients still relapse free at the end of analysis was 49.5 months (mean: 50.0 months).
Immunohistochemistry
Immunohistochemistry was performed using the streptavidin–biotin–peroxidase method with a Histofine streptavidin–biotin–peroxidase kit (Nichirei, Tokyo, Japan). The primary antibody used in this study were anti-GNMT antibody (dilution 1:50). Sections, 4 μm thick, from 10% formalin-fixed, paraffin-embedded material were deparaffinized in xylene and rehydrated through ethanol. Endogenous peroxidase activity was then blocked by methanol containing 0.3% hydrogen peroxidase for 30 min. After exposure to 10% nonimmunized goat serum in PBS for 10 min, the sections were incubated at 4°C overnight with the primary antibody. The sections were then incubated with the second antibody for 20 min. The reaction products were visualized by diaminobenzidine tetrahydrochloride as a chromogen. Finally, the sections were counterstained with hematoxylin.
Immunohistochemical Analysis
To assess the GNMT expression, we evaluated cytoplasmic and nuclear staining. The cytoplasmic expression levels of GNMT were semi-quantitatively scored using an immunoreactivity score; a combined score obtained from the values of the mean staining intensity and the percentage of positive staining cells. The percentage score represented as follow: 0, negative; 1, <10%; 2, 10–25%; 3, 26–50%; 4, 51–75%; 5, >75%. The intensity score represented as follow: 0, none; 1, weak; 2, moderate; 3, strong. Finally, high cytoplasmic expression of GNMT was defined as an immunoreactivity score of >5.
For scoring GNMT nuclear expression, tumors were considered positive when >10% of cells showed nuclear expression.
Statistical Analysis
We used Fisher's extact test and the χ2 test for statistical analysis of the correlations between immunohistochemical GNMT expression and the clinicopathologic parameters. Disease-free survival was taken as the time between surgery and the date of the last follow-up or PSA recurrence by disease. The survival curve was calculated by the Kaplan–Meier method, and the significance was analyzed by log-rank test. The Cox proportional hazards model was used for multivariate survival analysis, in which we estimated the following variables: GNMT expression, age, pre-operative PSA concentration, Gleason score, pT stage, and surgical margin. P-values <0.05 were considered significant.
Results
GNMT Expressions Are more Abundant in Prostate Cancer Cells Compared with Normal Prostate Epithelial Cells
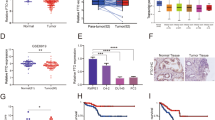
To investigate the relevance of GNMT in prostate cancer, we performed western blotting analysis using normal prostate epithelial cells and a panel of prostate cancer cells. GNMT expression was upregulated in most PCa cells, especially in PC-3 and LNCaP, compared with normal prostate epithelial cells (RWPE-1) (Figure 1a).
(a) GNMT expression was more abundant in prostate cancer cells than in normal prostate epithelial cells. Whole-cell extracts of RWPE-1 and a panel of PCa cells were subjected to SDS–PAGE, and western blotting was performed using the indicated antibodies. (b) GNMT knockdown successfully suppressed GNMT expression. Whole-cell extracts were prepared from RWPE-1, PC-3, and LNCaP cells transfected with 40 nmol/l of control siRNA, GNMT siRNA #1, or GNMT siRNA #2, and incubated for 72 h. Western blotting was performed using the indicated antibodies. (c) GNMT knockdown suppresses prostate cancer cell proliferation. RWPE-1, PC-3, and LNCaP cells were transfected with 40 nmol/l of control siRNA, GNMT siRNA #1, or GNMT siRNA #2 and incubated. The number of cells was counted at the indicated times. The results were normalized to the number of cells at 0 h. All values represent at least three independent experiments. Boxes, mean; bars, ±s.d.
GNMT Knockdown Suppresses Prostate Cancer Cell Proliferation and Induces Cellular Apoptosis in Prostate Cancer Cells
To investigate the role of GNMT in prostate cancer cell growth, we performed GNMT knockdown and counted the cell numbers after culture for the indicated times. First, we confirmed the efficiencies of two kinds of GNMT-specific siRNA by western blot analysis. As shown in Figure 1b, GNMT-specific siRNA successfully suppressed GNMT expression in RWPE-1, PC-3, and LNCaP cells. Using these GNMT-specific siRNAs, we found that cell proliferation of both PC-3 and LNCaP cells transfected with GNMT-specific siRNAs was apparently decreased, while suppression of GNMT expression induced little effect on RWPE-1 cell growth (Figure 1c). These results led us to carry out flow cytometry analyses to investigate the mechanism by which GNMT knockdown retarded cell growth and decreased the cell number in prostate cancer cells, especially in PC-3 cells. Silencing of GNMT expression in RWPE-1 cells led to slight increases in the G1 fraction (Figure 2a). In contrast, GNMT knockdown in PC-3, androgen-independent prostate cancer cells, induced prominent increases in the sub-G1 fraction, indicating cellular death (Figure 2b). In addition, suppression of GNMT expression in LNCaP, androgen-dependent prostate cancer cells, also exhibited moderate increases in sub-G1 and G1 fractions (Figure 2c). To investigate the mechanism of cellular death by GNMT silencing, immunoblotting using an antibody against cleaved PARP, which indicates the presence of degradation products from the caspase cascade, was used. After GNMT knockdown, RWPE-1, PC-3, and LNCaP cells were subjected to analysis. GNMT knockdown induced cleavage of PARP in prostate cancer cells, especially in PC-3 cells, but not in REPW-1 cells (Figure 2d).
GNMT knockdown induces cellular apoptosis in prostate cancer cells. (a–c) RWPE-1 (a), PC-3 (b), and LNCaP (c) cells were transfected with 40 nmol/l of control siRNA, GNMT siRNA #1, or GNMT siRNA #2. At 72 h post-transfection, cells were stained with propidium iodide and analyzed by flow cytometry. The cell-cycle fraction is shown in the upper right position of each graph. (d) The membrane used in Figure 1b was stripped and immunoblotted with the indicated antibodies.
GNMT Expression in the Cytoplasm and Nucleus of Prostate Epithelial Cells and Cancer Cells
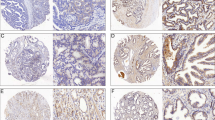
GNMT expression was vague level in the cytoplasm of normal prostatic epithelium (Figure 3a, left panel). The cytoplasmic expression was weak in Gleason pattern 3 prostate cancer (Figure 3a, right panel) and strong in Gleason pattern 5 (Figure 3b, left panel). GNMT expression was scattered in the nucleus of prostate cancer (Figure 3b, right panel). The cytoplasmic and nuclear expression were significantly higher in the cancer than the benign glands (P<0.001; Table 2).
Immunohistochemical staining of GNMT in human prostate tissue. (a) (Left panel) GNMT expression in normal prostatic epithelium; the levels of cytoplasmic and nuclear expression were vague in luminal epithelial cells. (a) (Right panel) GNMT expression in Gleason pattern 3 prostate cancer; weak cytoplasmic expression was observed. (b) (Left panel) GNMT expression in Gleason pattern 5 prostate cancer; strong cytoplasmic expression was observed. (b) (Right panel) Nuclear expression of GNMT in Gleason pattern 3 prostate cancer. Cytoplasmic expression was also recognized. (c) Disease-free survival curves of patients in low and high GNMT cytoplasmic expression groups.
Clinicopathological Parameters and Disease-Free Survival Analysis
We have summarized the correlations between GNMT expression and clinicopathological parameters in Table 3. Higher GNMT cytoplasmic expression was associated with a higher Gleason score (P<0.001) and higher pT stage (P=0.027). Nuclear expression of GNMT was not associated with pre-operative PSA concentration, Gleason score, or stage.
PSA recurrence was found in 31.4% (22/70) of individuals with high GNMT cytoplasmic expression, whereas PSA recurrence was found in only 6.9% (4/58) of individuals with low GNMT cytoplasmic expression. Patients with high GNMT cytoplasmic expression had lower disease-free survival rates compared with the low-expression group (log-rank test, P<0.001; Figure 3c). The difference was not significant between the negative and positive nuclear expression groups (log-rank test, P=0.745). The Cox proportional hazards model revealed that high GNMT cytoplasmic expression (P=0.005), high PSA level (P<0.001), and high-grade Gleason (P=0.011) are independent prognostic factors (Table 4).
Discussion
To our knowledge, this is the first analysis to show that the GNMT is linked with prostate cancer proliferation and contributes to carcinogenesis and progression of prostate cancer.
We have found that siRNA-mediated GNMT knockdown results in complete inhibition of the proliferation of cancer cell lines but minimal inhibition of benign cell line (Figure 1c). Furthermore, flow cytometry analysis showed that silencing of GNMT expression by siRNA in prostate cancer cells induces a prominent increase in the sub-G1 fraction (Figure 2b). GNMT knockdown induced cleavage of PARP in prostate cancer cells (Figure 2d). The increase in the sub-G1 fraction and the induction of cleavage of PARP were more prominent in more aggressive, androgen-independent prostate cancer cell lines PC-3 than in less aggressive, androgen-dependent LNCaP. PARP helps cells to maintain their viability. In contrast, cleavage of PARP facilitates cellular disassembly and serves as a marker of cells undergoing apoptosis.17 These findings indicate that the silencing of GNMT expression by siRNA induces apoptosis in more aggressive prostate cancer cells. GNMT may have an important role in promoting prostate cancer cell growth via the regulation of apoptosis. Our results seem to disagree with previous findings that GNMT is a tumor susceptibility gene,10, 11, 12 but agree with the previous findings that GNMT activity is linked with the progression of cancer.5
According to our findings, we hypothesize that GNMT expression may correlate with malignant progression and prognosis. We therefore examined whether immunohistochemical GNMT expression is correlated with progression and prognosis in prostate cancer tissues.
Sixteen tissues from different human organs showed by northern blot analysis of 16 tissues from different human organs showed that GNMT was expressed only in the liver, pancreas, and prostate.18 This group has reported that GNMT expression is localized in the cytoplasm of hepatocytes19 and in both the cytoplasm and nucleus of prostate epithelium.12 Their findings are consistent with our immunohistochemical data, indicating that GNMT expression is predominantly located in the cytoplasm and scattered in the nuclei of prostate cells.
Huang et al12 have reported that immunohistochemical results show abundant GNMT expression in normal prostatic and benign prostatic hyperplasia tissues, whereas its expression is diminished in prostate cancer tissues. In our study, GNMT expression was predominantly low in normal prostatic tissues, whereas it was predominantly high in the prostate cancer tissues (Table 2). In addition, the cytoplasmic expression of GNMT contributes to a higher Gleason score, a higher pT stage, and a prognosis of disease-free survival (Tables 3 and 4; Figure 3c). Our results support the finding that sarcosine, which is generated from glycine through the enzymatic activity of GNMT, could potentially be used for the diagnosis and prediction of prognosis in prostate cancer.5
One might ask why GNMT expression differentially affected prostate normal epithelium and cancer in the studies by both Huang et al12 and us. The reasons for this discrepancy remain unclear at present. However, our western blotting data showed that GNMT expression is more abundant in prostate cancer cell lines than in a normal prostate epithelial cell line (Figure 1a). This finding is consistent with our immunohistochemical data. Furthermore, the specificity of GNMT antibody in our study was demonstrated, as GNMT-specific siRNA successfully suppressed GNMT expression in normal prostate and cancer cells by western blotting (Figure 1b). This discrepancy might have been caused by the different interpretation of immunohistochemical results. They evaluated only the cytoplasmic expression of GNMT,12 while we evaluated both the cytoplasmic and the nuclear expression, respectively.
The nuclear expression of GNMT was more abundant in prostate cancer compared with normal prostate tissue (Table 2), thus indicating that GNMT nuclear expression may be linked with carcinogenesis in the prostate. However, its nuclear expression was not associated with age, Gleason score, stage, or pre-operative PSA concentration. The specific function of the nuclear localization of GNMT is unknown at present. Further analysis will clarify the specific function of the nuclear localization of GNMT.
In conclusion, our findings demonstrate that GNMT may have an important role in promoting prostate cancer cell growth via the regulation of apoptosis. The modulation of GNMT expression or function may provide a strategy for developing novel therapeutics for prostate cancer. Furthermore, GNMT may represent a novel marker of malignant progression and poor prognosis in prostate cancer.
References
Parkin DM . Global cancer statistics in the year 2000. Lancet Oncol 2001;2:533–543.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66.
Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 2003;169:517–523.
Abate-Shen C, Shen MM . Diagnostics: the prostate-cancer metabolome. Nature 2009;457:799–800.
Sreekumar A, Poisson LM, Rajendiran TM, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009;457:910–914.
Kerr SJ . Competing methyltransferase systems. J Biol Chem 1972;247:4248–4252.
Cook RJ, Wagner C . Measurement of a folate binding protein from rat liver cytosol by radioimmunoassay. Arch Biochem Biophys 1981;208:358–364.
Yeo EJ, Wagner C . Tissue distribution of glycine N-methyltransferase, a major folate-binding protein of liver. Proc Natl Acad Sci USA 1994;91:210–214.
Liu HH, Chen KH, Shih YP, et al. Characterization of reduced expression of glycine N-methyltransferase in cancerous hepatic tissues using two newly developed monoclonal antibodies. J Biomed Sci 2003;10:87–97.
Tseng TL, Shih YP, Huang YC, et al. Genotypic and phenotypic characterization of a putative tumor susceptibility gene, GNMT, in liver cancer. Cancer Res 2003;63:647–654.
Martinez-Chantar ML, Vazquez-Chantada M, Ariz U, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology 2008;47:1191–1199.
Huang YC, Lee CM, Chen M, et al. Haplotypes, loss of heterozygosity, and expression levels of glycine N-methyltransferase in prostate cancer. Clin Cancer Res 2007;13:1412–1420.
Huang YC, Chen M, Shyr YM, et al. Glycine N-methyltransferase is a favorable prognostic marker for human cholangiocarcinoma. J Gastroenterol Hepatol 2008;23:1384–1389.
Shiota M, Izumi H, Onitsuka T, et al. Twist promotes tumor cell growth through YB-1 expression. Cancer Res 2008;68:98–105.
Shiota M, Izumi H, Onitsuka T, et al. Twist and p53 reciprocally regulate target genes via direct interaction. Oncogene 2008;27:5543–5553.
Shiota M, Izumi H, Tanimoto A, et al. Programmed cell death protein 4 down-regulates Y-box binding protein-1 expression via a direct interaction with Twist1 to suppress cancer cell growth. Cancer Res 2009;69:3148–3156.
Oliver FJ, de la Rubia G, Rolli V, et al. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem 1998;273:33533–33539.
Chen YM, Chen LY, Wong FH, et al. Genomic structure, expression, and chromosomal localization of the human glycine N-methyltransferase gene. Genomics 2000;66:43–47.
Chen YM, Shiu JY, Tzeng SJ, et al. Characterization of glycine-N-methyltransferase-gene expression in human hepatocellular carcinoma. Int J Cancer 1998;75:787–793.
Acknowledgements
We thank Dr Dongchon Kang (Kyushu University, Fukuoka, Japan) and Dr Takeshi Uchiumi (Kyushu University) for helping with flow cytometry. The English used in this manuscript was revised by KN International (3 Golf Center, Suite #407, Hoffman Estates, IL 60169, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Song, Y., Shiota, M., Kuroiwa, K. et al. The important role of glycine N-methyltransferase in the carcinogenesis and progression of prostate cancer. Mod Pathol 24, 1272–1280 (2011). https://doi.org/10.1038/modpathol.2011.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.76
Keywords
This article is cited by
-
In Silico Prediction of Metabolic Fluxes in Cancer Cells with Altered S-adenosylmethionine Decarboxylase Activity
Cell Biochemistry and Biophysics (2021)
-
The non-canonical mechanism of ER stress-mediated progression of prostate cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Molecular signatures associated with prostate cancer cell line (PC-3) exposure to inactivated Zika virus
Scientific Reports (2019)
-
Prostate tumor attenuation in the nu/nu murine model due to anti-sarcosine antibodies in folate-targeted liposomes
Scientific Reports (2016)
-
Implications of differences in expression of sarcosine metabolism-related proteins according to the molecular subtype of breast cancer
Journal of Translational Medicine (2014)