Abstract
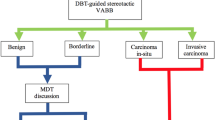
The necessity of excision is debatable when atypia are diagnosed at stereotactic vacuum-assisted breast biopsy (microbiopsy). Among the 287 surgical excisions performed at Institut Bergonié from 1999 to 2009, we selected a case–control study group of 151 excisions; 52 involving all the diagnosed cancers and 99 randomly selected among the 235 excisions without cancer, following atypical microbiopsy (24 flat epithelial atypia; 50 atypical ductal hyperplasia; 14 lobular neoplasia; 63 mixed lesions). Mammographical calcification (type, extension, complete removal) and histological criteria of epithelial atypia (type, number of foci, size/extension), topography and microcalcification extension at microbiopsy were compared according to the presence or absence of cancer at excision. Factors associated with cancer at excision were Breast Imaging Reporting and Data System (BI-RADS5) lesions, large and/or multiple foci of mammographical calcifications, histological type, number, size and extension of atypical foci. Flat epithelial atypia alone was never associated with cancer at excision. BI-RADS5, atypical ductal hyperplasia (alone or predominant) and >3 foci of atypia were identified as independent pejorative factors. There was never any cancer at excision when these pejorative factors were absent (n=31). Presence of one (n=59), two (n=23) or three (n=14) factors was associated with cancer in 24, 15 and 13 cases with an odds ratio=5.8 (95% CI: 3–11.2) for each additional factor. We recommend that mammographical data and histological characteristics be taken into account in the decision-making process after diagnosis of atypia on microbiopsy. With experienced senologists and strict histological criteria, some patients could be spared surgery resulting in significant patient, financial and time advantages.
Similar content being viewed by others
Main
More and more stereotactic vacuum-assisted breast biopsies (microbiopsies) are being performed in routine practice on infraclinical lesions. In a previous study on 2833 surgical biopsies performed for isolated microcalcifications without any palpable tumors, epithelial atypia were found adjacent to small, low-grade concomitant cancer in 30% of cases.1 This would indicate that excision is more often not warranted, but there are still no clear guidelines for the management of patients when epithelial atypia are diagnosed at microbiopsy. Up until now, most reports published in the literature2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 have focused on underestimation of lesions at microbiopsy when compared with surgical excisions. Some authors have identified patients who should or should not be spared surgery when epithelial atypia were diagnosed at microbiopsy performed for microcalcifications, but criteria to excise or not differ according to the authors.12, 14, 15, 16 Jackman et al7 report that no clinical, mammographic or biopsy features alone or in combination can be used to define a substantial subset of probably benign lesions with a <2% chance of cancer at surgery. In all these studies, the number of microbiopsies performed was far greater than the number of subsequent surgical excisions (from 31 (11) to 116 (12)) and only small numbers of cancer diagnosed (3 (8) to 29 (12)). El-Sayed et al17 evaluated the positive predictive values for malignancy at surgery according to the type of benign and/or atypical so-called B3 lesions (n=523) at microbiopsy. Among B3 lesions, there were 147 atypical ductal hyperplasia and 27 lobular neoplasia including lobular carcinoma in situ with, respectively, 49 and 8 cancers at excision. One reason why previous results must be interpreted with caution, and which potentially explains the discrepancies in results observed, is that interobserver reproducibility in the classification of radiological lesions remains at present low18 to moderate,19 especially for non-experienced readers20 and it is the same for histological lesions21 since distinguishing between flat epithelial atypia and atypical ductal hyperplasia is sometimes difficult using the WHO criteria.22 Furthermore, the distinction between atypical ductal hyperplasia and low-grade ductal carcinoma in situ on a core biopsy specimen is uncertain since the extent of atypia cannot be accurately assessed. Consequently, as already highlighted by Jacobs et al,23 controversies still remain between those who think that excision is warranted because they are not able to predict with confidence which patients will not have an associated breast cancer, and those who currently do not excise if the most significant lesion is flat epithelial atypia.
The aim of our study is to account for these issues by defining criteria on which to base the decision of whether or not to excise when atypia are diagnosed by microbiopsy, by comparing mammographical data (with experienced radiologists) and histological characteristics (with strict criteria) at microbiopsy according to the concomitant presence or absence of cancer in a large case–control group of surgical excisions.
Materials and methods
Selection of the Case–Control Study Group
From January 1999 to January 2009, 1563 11-gauge microbiopsies were performed for microcalcifications at Institut Bergonié and 335 cases of epithelial atypia were diagnosed (21.4% of total biopsies). Two hundred and eighty seven (86%) had surgery at our institution and 48 outside (14%). There were 52 (18%) and 235 (82%) surgical excisions with and without cancer, respectively. The aim of this study was to identify risk factors on microbiopsy associated with the presence of cancer in surgical excisions. The use of a case study group was selected so as to minimize cost and time of the study, given that this design using an odds ratio (OR) fully meets the study objectives. Thus, the study group comprised of the 52 cases with cancer and a subset of 100 randomly selected cases among the 235 surgical excisions without cancer. The 152 microbiopsies were centrally reviewed by the same senior pathologist (IM). One case was excluded since lesions were reclassified as non-atypical. Thus, for this case–control study, 151 cases were available. Surgical biopsies were macroscopically serially sectioned in their entirety into numbered slices every 2 mm (median number of blocks per re-excision: 26, min–max: 1–44). Cancers on excision were classified according to the WHO classification22 and corresponded to in situ and invasive carcinomas in 40 and 12 cases, respectively. Mean sizes of in situ and invasive carcinomas were 11.3 mm (2–50) and 5 mm (1–13), respectively. The mean age of the study group was 54.5 years (32–79).
Mammographical Characteristics of Microcalcifications
Prebiopsy mammograms with microcalcifications were reviewed by two experienced radiologists (MAS and GH) using the Breast Imaging Reporting and Data System (BI-RADS) 4th edition lexicon (Table 1).24 The number of foci was assessed and when there was only one focus (n=118), we examined the size of the area of microcalcifications. It was ≤20 mm in 92 cases and >20 mm in 26 cases; not specified in 19 cases. The number of cores was recorded by the radiologist (median: 12). A radiography of these specimens was systematically performed and transmitted to the pathologist with core specimens. In each case, core specimens were separated according to the presence or the absence of microcalcifications. Furthermore, the radiologist specified if the microcalcifications had been removed in toto (n=46) or not (n=102); not specified in three cases.
Examination of Core Specimens
Because of the macroscopic fragmentation, the number of cores counted by the pathologist was higher than the one counted by the radiologist: median 19 (3–43). After fixation, core specimens with and without microcalcifications were put separately in as many numbered cassettes as necessary and embedded in paraffin: median of seven blocks (from 1 to 22) per microbiopsy. In most cases (90%), one hematoxylin and eosin-stained slide per block was performed. When difficulties in diagnosis were encountered (non-atypical vs atypical ductal hyperplasia, atypical ductal hyperplasia vs lobular neoplasia, invasive vs in situ carcinoma), recuts of the blocks and/or immunohistochemical stainings with cytokeratin 5/6 (positive in non-atypical epithelial proliferation) and/or E-Cadherin (negative in lobular epithelial hyperplasia) were performed (number of cases not recorded). Histological characteristics are summarized in Table 1.
Microcalcifications
After fixation, the extension (number of cores and/or the percentage of blocks with calcifications) and topography of microcalcifications in and out of epithelial atypia, were also examined according to the type of epithelial atypia.
Types of atypia
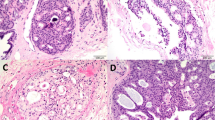
Epithelial atypia at microbiopsy were classified according to the WHO 2003 classification22 as flat epithelial atypia flat epithelial aytpia (Figure 1a and b), atypical ductal hyperplasia (Figure 2a and b) and lobular neoplasia type 1 or 2 (Figure 3a and b). There was never more than one micropapillation, arcade or trabecular bar in any duct with flat epithelial atypia, and each focus of atypical ductal hyperplasia sized ≤3 mm.25 In the present study, four subgroups of epithelial atypia were identified: the group with flat epithelial atypia only (n=24), the group with atypical ductal hyperplasia only (n=50), the group of lobular neoplasia only (n=14) and the group with mixed lesions (n=63).
Size and extension of epithelial atypia
At each microbiopsy, the number of foci with epithelial atypia, the diameter of the largest focus, the sum of sizes of all different foci, the number of core specimens with epithelial atypia and the percentage of cores with epithelial atypia, that is, the number of core specimens with atypia vs the total number of core specimens, were assessed according to the type of epithelial atypia. In the 63 mixed cases, the sum of the sizes of foci for each lesion was taken into account. A lesion was recorded as predominant when this sum was larger than the sum of the sizes of foci of the other associated lesion(s). Flat epithelial atypia, atypical ductal hyperplasia and lobular neoplasia were predominant in 35, 18 and 7 cases, respectively. Predominant flat epithelial atypia lesions were associated with atypical ductal hyperplasia, lobular neoplasia and atypical ductal hyperplasia + lobular neoplasia in 20, 5 and 10 cases, respectively. Predominant atypical ductal hyperplasia lesions were associated with flat epithelial atypia, flat epithelial atypia + lobular neoplasia and lobular neoplasia in 7, 3 and 8 cases, respectively. Predominant lesions of lobular neoplasia were associated with flat epithelial atypia and atypical ductal hyperplasia + lobular neoplasia in 5 and 2 cases, respectively. In three cases, there was no predominance (two cases with flat epithelial atypia and atypical ductal hyperplasia and one case with flat epithelial atypia and lobular neoplasia). When associated, flat epithelial atypia and atypical ductal hyperplasia corresponded either to separated foci of flat epithelial atypia and atypical ductal hyperplasia or to small foci of atypical ductal hyperplasia within flat epithelial atypia. In this last eventuality (n=14), atypical ductal hyperplasia corresponded to a varying number of micropapillations at the periphery of ducts with flat epithelial atypia (Figure 2b).
Statistical Analyses
Comparisons of these clinical and histological characteristics from microbiopsy according to the presence or absence of cancer on re-excision were performed with logistic regression. Only odds and OR can be reported.26 Multivariate analysis was performed using a logistic regression model. P-values of <0.05 were considered statistically significant.
Results
In this section, we will look at predictive factors of cancer at excision in our series, which included 100 cases without cancer and 52 cases with cancer (40 in situ and 12 invasive carcinomas). Among cancers in situ there were 36 low-grade ductal carcinoma in situ (28 atypical ductal hyperplasia, two lobular neoplasia and six atypical ductal hyperplasia plus lobular neoplasia at microbiopsy, respectively), and four lobular carcinoma in situ (three lobular neoplasia and one atypical ductal hyperplasia plus lobular neoplasia at microbiopsy, respectively). Among invasive cancers, there were one tubular carcinoma with atypical ductal hyperplasia at microbiopsy, two invasive lobular carcinomas grade II (one lobular neoplasia and one atypical ductal hyperplasia plus lobular neoplasia at microbiopsy) and nine invasive ductal carcinomas grade I (seven atypical ductal hyperplasia and two atypical ductal hyperplasia plus lobular neoplasia at microbiopsy, respectively).
Cancer at Re-excision According to Mammographical Characteristics
Cancer at re-excision was more frequently associated at microbiopsy with BI-RADS5 than with BI-RADS3 or BI-RADS4 lesions (OR=4.4; 95% CI: 1.8–11.2; P=2 × 10−3), and with larger (>20 mm), lesions and/or multiple foci of microcalcifications than small, singular focus (OR=2.3; 95% CI: 1.1–4.9; P=3 × 10−2) (Table 2). No association was observed between the disappearance of the mammographical lesion after microbiopsy and cancer.
Cancer at Re-excision According to Histological Characteristics at Microbiopsy
Type of atypia
Flat epithelial atypia as a single lesion at microbiopsy was never associated with cancer at re-excision (Table 2). Atypical ductal hyperplasia as a single lesion was more frequently associated with cancer than lobular neoplasia (OR=6.5; 95% CI: 1.6–26.4; P=9 × 10−3) or mixed lesions (OR=4.8; 95% CI: 2.2–10.7; P=1.2 × 10−4). Six out of the 14 cases with atypical ductal hyperplasia corresponding to a varying number of micropapillations at the periphery of ducts with flat epithelial atypia were associated with cancer. Six out of the nine cases of pure and predominant lobular neoplasia with calcifications located within lobular neoplasia lesions had cancer at excision. In contrast, none of the pure and predominant lesions of lobular neoplasia without calcifications in lobular neoplasia lesions (n=12) had cancer at excision.
Size/extension of atypia
Cancer at excision was more likely to be observed as the number (OR=1.2; 95% CI: 1.1–1.3; P=1.5 × 10−3) and percentage (OR=1.02; 95% CI: 1.01–1.03; P=1.6 × 10−3) of core specimens with epithelial atypia increased and as the number (OR=1.2; 95% CI: 1.1–1.4; P=2.8 × 10−4) and sum of the sizes (OR=1.1; 95% CI: 1.02–1.1; P=7 × 10−3) of atypical foci increased.
Microcalcifications
Cancer at re-excision was not associated with the number of core specimens with calcifications nor with the topography of calcifications (related to epithelial atypia or not).
When taking into account lesions of atypical ductal hyperplasia and lobular neoplasia only, cancer at excision was more likely to be observed after microbiopsy with atypical ductal hyperplasia alone or predominant (OR=4.8; 95% CI: 2.2–1.04; P=9 × 10−5), and as the number (OR=1.3; 95% CI: 1.1–1.5; P=4.6 × 10−4) and percentage (OR=1.04; 95% CI: 1.2–1.05; P=1.6 × 10−4) of core specimens with epithelial atypia increased and as the number (OR=1.4; 95% CI: 1.2–1.7; P=6.7 × 10−5), largest size (OR=1.4; 95% CI: 1.01–1.9; P=4 × 10−2) and sum of the sizes (OR=1.2; 95% CI: 1.1–1.4; P=1.4 × 10−4) of atypical foci increased.
By multivariate analysis including BI-RADS classification, size of the radiological lesion, type (lesions of flat epithelial atypia excluded), sizes, number of foci of epithelial atypia, number and percentage of core specimens with epithelial atypia and presence of calcifications in atypia, three criteria were identified as independent factors predictive of cancer at excision (Table 3): >3 foci of atypia (OR=9.6; 95% CI: 1.2–12.2; P=3 × 10−2), atypical ductal hyperplasia (alone or predominant) (OR=5.2; 95% CI: 2–13.1; P=6.1 × 10−4), and BI-RADS5 (OR=3.7; 95% CI: 1.2–12.2; P=3 × 10−2). There was never any cancer at excision when these pejorative factors were absent (n=31). Presence of one (n=59), two (n=23) or three (n=14) factors was associated with cancer in 24, 15 and 13 cases with an OR=5.8 (95% CI: 3–11.2) for each additional factor.
Discussion
Criteria in the Literature on Which to Decide Whether or not to Excise
In the present study, flat epithelial atypia was never associated with cancer at excision, and this result is the same in Senetta et al’ series15 of 38 flat epithelial atypia. Jara-Lazaro et al16 report from small population groups the presence of ductal carcinoma and lobular neoplasias in excision biopsies for up to 22 and 36% of core biopsies with pure flat epithelial atypia. Micropapillary lesions corresponding to a varying number of micropapillations at the periphery of ducts with flat epithelial atypia are in most studies included in the group of atypical ductal hyperplasia. In our study, as in Ely et al's5 and Wagoner et al's14 reports, these microapapillary lesions were associated in some cases with cancer at surgery. When considering atypical ductal hyperplasia lesions, our data are discordant. Taking into account the size of atypical ductal hyperplasia lesions (cutoffs at 6 and 21 mm) and removal of microcalcifications, Forgeard et al12 identified three groups of patients with atypical ductal hyperplasia on 11-gauge microbiopsy (n=116) and cancer at excision (n=29) according to the incidences of cancer in each group. In the first group (n=17), there was no cancer at surgery. In the second group, there was 1/24 cancer (4%) and in the third group there was 28/75 (37%) cancers. They concluded that in the first two groups, strict follow-up can be a safe option and in the third group, surgery is mandatory. Applying their criteria in our series, cancer at surgery would be found in 5, 5 and 36 cases in the three groups, respectively. The difference observed in our series may be explained by our selected case–control study group involving all cancers at re-excision at our Institute from 1999 to 2009. To our knowledge, our study group represents the largest number of surgical excisions with cancer following microbiopsy epithelial atypia. In the same way, Wagoner et al14 identified one group of patients with atypical ductal hyperplasia at microbiopsy and no cancer at surgery (n=25). In this group, there were less than three atypical ductal hyperplasia foci, microcalcifications were located in atypical ductal hyperplasia and the mammographic abnormality was completely removed. Applying these criteria in our series, cancer at surgery would be found in two patients. Furthermore, discordances may also be explained by still elusive definitions of lesions as underlined below.
Interobserver Reproducibility in the Classification of Epithelial Atypia
In the WHO classification,22 flat epithelial atypia is characterized by replacement of the native epithelial cells by a single layer of mildly atypical cells or proliferation of a monotonous atypical cell population with occasional mounding. Arcades and micropapillary formations are absent or very rare. When such architectural atypia are present in lesions of flat epithelial atypia, the problem is defining where the diagnosis of flat epithelial atypia ends and atypical ductal hyperplasia begins.16 This explains difficulties in separating flat epithelial atypia with ‘very rare’ (how many?) micropapillary formations or ‘occasional’ (how many?) moundings, from atypical ductal hyperplasia. This is why we individualized atypical ductal hyperplasia with micropapillary architecture in the group of mixed lesions. With strict criteria, that is, none,15 or one, micropapillation/cribriform space in one or several ducts with flat epithelial atypia (as in the present study), no cancer was found at excision. Micropapillary lesions seem to be an intermediate step between flat epithelial atypia and pure non-micropapillary atypical ductal hyperplasia with 43 and 61% of cancers at surgery. As stated by Simpson,27 there is a morphological and molecular continuum in the degree of proliferation and atypia, and all these lesions are a non-obligate, intermediary step in the development of low-grade carcinomas. Our results confirm this non-obligate continuum since when low-grade ductal carcinoma in situ or infiltrating carcinomas were present at excision, atypical ductal hyperplasia was present at microbiopsy in almost (34 out of 36) or all the cases (n=10), respectively. In the same way, it is interesting to note that the two invasive lobular carcinomas were associated with lobular neoplasia at microbiopsy. Flat epithelial atypia represents the earliest morphologically recognizable neoplastic alteration of the breast that is commonly associated with mammographically suspicious microcalcifications.28 Atypical ductal hyperplasia is recognized either on qualitative criteria, recognition of some but not all features of ductal carcinoma in situ, corresponding to ‘mimicking’ ductal carcinoma in situ, or on quantitative criteria, corresponding to ‘mini ductal carcinoma in situ’ sized ≤3 mm.1, 25 It should be noted however that interobserver diagnostic reproducibility concerning flat epithelial atypia and atypical ductal hyperplasia is low as we have already shown with respective values of 0.44 and 0.4321 with difficulties differentiating flat epithelial atypia from columnar cell lesions without atypia and atypical ductal hyperplasia from ductal carcinoma in situ. However, the results of the present study are encouraging in the sense that differentiating columnar cell lesions from pure flat epithelial atypia at microbiopsy does not change patient management, that is, follow-up and no excision.
Interobserver Reproducibility in the Classification of Radiological Lesions
In Senetta et al's study,15 BI-RADS5 lesions were never associated with flat epithelial atypia (5/24 in our series). This result raises the problem of interobserver reproducibility in the classification of radiological calcifications. Using the BI-RADS lexicon 4th edition,24 some authors have evaluated the interobserver variability in, respectively, 83 and 94 breast lesions with calcifications.18, 19 The overall diagnostic agreement was low19 to moderate18 but improved in the BI-RADS5 category18, 19 and with experienced readers.20
Conclusions for the Management of Patients According to the Number of Pejorative Criteria and the Type of Atypia
Our results enable us to categorize patients into three groups based on the presence or absence of pejorative factors predictive of resulting cancer to inform the decision whether or not to excise.
Not to excise
Patients with strict criteria of flat epithelial atypia or with none of the three pejorative factors (BI-RADS5, atypical ductal hyperplasia alone or predominant and/or >3 foci of atypia), could be spared surgery, resulting in significant savings for the patient in terms of surgical comorbidities, in time and financial. At our institution, $2296USD per case would be saved.
To excise
Our results indicate that lesions with two or three pejorative independent factors at microbiopsy must be excised.
To excise or not to excise?
Difficulties remain in patients with only one pejorative factor. When the only one pejorative factor is a BI-RADS5 lesion and if the atypical/non-atypical benign histological lesion is discordant with the mammography, the sampling quality must be discussed with senologists. When the only pejorative factor corresponds to ≤3 foci of atypical ductal hyperplasia alone or to >3 foci of non-predominant lesions of atypical ductal hyperplasia associated with lobular neoplasia, the necessity of the excision is debatable and will be discussed with senologists, taking into account the radiological criteria (size, disappearance of microcalcifications) and histological characteristics (number, type and size of atypical foci). When the only pejorative factor is the presence of >3 foci of pure or predominant lobular neoplasia1/lobular neoplasia type 2, the necessity of the excision is debatable according to whether lobular neoplasia lesions are considered as incidental or correspond to the targeted lesions. In our study, pure (n=5 cases) and predominant (n=4) lobular neoplasia lesions with calcifications within lobular neoplasia, most likely corresponded to the targeted lesions while pure (n=9) and predominant (n=3) lobular neoplasia lesions without calcifications, that is, located in concomitant benign lesions, probably corresponded to incidental lesions. Three out of the five pure cases and three out of the four predominant cases of lobular neoplasia lesions with calcifications within lobular neoplasia lesions had cancer at excision, respectively. In fact, all pure and predominant lesions of lobular neoplasia on microbiopsy with cancer at excision had >3 foci of NL. In contrast, none of the pure (n=9) and predominant (n=3) lesions of lobular neoplasia without calcifications in lobular neoplasia lesions had cancer at excision. Excision is necessary when >3 foci of lobular neoplasia1/lobular neoplasia type 2 with calcifications located within lesions are found on microbiopsy. Last, when there are >3 foci of non-predominant lesions of atypical ductal hyperplasia and lobular neoplasia, in association with flat epithelial atypia and BI-RADS5 lesions, the number of pejorative factors may be difficult to assess since some flat epithelial atypia (five in our study) correspond to BI-RAD5 lesions. In these cases, topography of calcifications within each histological type of lesion has to be assessed with accuracy by pathologists and discussed with senologists.
In conclusion, with mammographical criteria and strict histological criteria, our method enables the identification of three groups of patients: the group who could be spared surgery, resulting in significant savings, the group of patients who should be excised and the last group for whom excision has to be discussed by confirmed experienced senologists and pathologists.
References
de Mascarel I, MacGrogan G, Mathoulin-Pélissier S, et al. Epithelial atypia in biopsies performed for microcalcifications. Practical considerations about 2833 serially sectioned surgical biopsies with a long follow-up. Virchows Arch 2007;451:1–10.
Adrales G, Turk P, Wallace T, et al. Is surgical excision necessary for atypical ductal hyperplasia of the breast diagnosed by Mammotome? Am J Surg 2000;180:313–315.
Darling ML, Smith DN, Lester SC, et al. Atypical ductal hyperplasia and ductal carcinoma in situ as revealed by large-core needle breast biopsy: results of surgical excision. AJR Am J Roentgenol 2000;175:1341–1346.
Burak Jr WE, Owens KE, Tighe MB, et al. Vacuum-assisted stereotactic breast biopsy: histologic underestimation of malignant lesions. Arch Surg 2000;135:700–703.
Ely KA, Carter BA, Jensen RA, et al. Core biopsy of the breast with atypical ductal hyperplasia: a probabilistic approach to reporting. Am J Surg Pathol 2001;25:1017–1021.
Renshaw AA, Cartagena N, Schenkman RH, et al. Atypical ductal hyperplasia in breast core needle biopsies. Correlation of size of the lesion, complete removal of the lesion, and the incidence of carcinoma in follow-up biopsies. Am J Clin Pathol 2001;116:92–96.
Jackman RJ, Birdwell RL, Ikeda DM . Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology 2002;224:548–554.
Sneige N, Lim SC, Whitman GJ, et al. Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications. Considerations for surgical excision. Am J Clin Pathol 2003;119:248–253.
Winchester DJ, Bernstein JR, Jeske JM, et al. Upstaging of atypical ductal hyperplasia after vacuum-assisted 11-gauge stereotactic core needle biopsy. Arch Surg 2003;138:619–622.
Plantade R, Hammou JC, Fighiera M, et al. Underestimation of breast carcinoma with 11-gauge stereotactically guided directional vacuum-assisted biopsy. J Radiol 2004;85:391–401.
Travade A, Isnard A, Bouchet F, et al. Non-palpable breast lesions and core needle biopsy with Mammotome 11G: is surgery required in patients with atypical ductal hyperplasia? J Radiol 2006;87:307–310.
Forgeard C, Benchaib M, Guerin N, et al. Is surgical mandatory in case of atypical ductal hyperplasia on 11-gauge core needle biopsy? A retrospective study of 300 patients. Am J Surg 2008;196:339–345.
Sigal-Zafrani B, Muller K, El Khoury C, et al. Institut Curie Breast Cancer Study Group. Vacuum-assisted large-core needle biopsy (Vlobular neoplasiaB) improves the management of patients with breast microcalcifications: analysis of 1009 cases. Eur J Surg Oncol 2008;34:377–381.
Wagoner MJ, Laronga C, Acs G . Extent and histologic pattern of atypical ductal hyperplasia present on core needle biopsy specimens of the breast can predict ductal carcinoma in situ in subsequent excision. Am J Clin Pathol 2009;131:112–121.
Senetta R, Campanino PP, Mariscotti G, et al. Columnar cell lesions associated with breast calcifications on vacuum-assisted core biopsies: clinical, radiographic, and histological correlations. Mod Pathol 2009;22:762–769.
Jara-Lazaro AR, Tse GM, Tan PH . Columnar cell lesions of the breast: an update and significance on core biopsy. Pathology 2009;41:18–27.
El-Sayed ME, Rakha EA, Reed J, et al. Predictive value of needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening). Histopathology 2008;53:650–657.
Lazarus E, Mainiero MB, Schepps B, et al. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology 2006;239:385–391.
Coşar ZS, Cetin M, Tepe TK, et al. Concordance of mammographic classifications of microcalcifications in breast cancer diagnosis: Utility of the Breast Imaging Reporting and Data System (fourth edition). Clin Imaging 2005;29:389–395.
Ciatto S, Houssami N, Apruzzese A, et al. Reader variability in reporting breast imaging according to BI-RADS assessment categories (the Florence experience). Breast 2006;15:44–51.
MacGrogan G, Arnould L, de Mascarel I, et al. GEFPICS group. Impact of immunohistochemical markers, CK5/6 and E-cadherin on diagnostic agreement in non-invasive proliferative breast lesions. Histopathology 2008;52:689–697.
Tavassoli A, Devilee P (eds). WHO Classification of tumours: Pathology and Genetics. Tumours of the Breast and Female Genital Organs. IARC Press: Lyon, 2003, 432 pp.
Jacobs TW, Conolly JL, Schnitt SJ . Non malignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol 2002;26:1095–1110.
American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS), 4th edn. American College of Radiology: Restion, VA, 2003.
Pathology Reporting of Breast Disease. A Joint Document Incorporating the Third Edition of the NHS Breast Screening Programme’s. Guidelines for Pathology Reporting in Breast Cancer Screening and the Second Edition of the Royal College of Pathologsits’ Minimum Dataset for Breast Cancer Histopathology. Sheffield: NHS Cancer Screening Programmes (jointly with The Royal College of Pathologists, London), Publication 58, 2005, 134 p.
Rothman KJ, Greenland S, Lash TL (eds). Modern Epidemiology, 3rd edn. Lippincott William and Wilkins: Philadelphia, 2008, 851 pp.
Simpson PT, Gale T, Reis-Filho JS, et al. Columnar cell lesions of the breast: the missing link in breast cancer progression? A morphological and molecular analysis. Am J Surg Pathol 2005;29:734–746.
Moinfar F . Flat ductal intraepithelial neoplasia of the breast: a review of diagnostic criteria, differential diagnoses, molecular-genetic findings, and clinical relevance: it is time to appreciate the Azzopardi concept!. Arch Pathol Lab Med 2009;133:879–892.
Acknowledgements
We thank Pippa McKelvie-Sebileau for her help with the English version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
de Mascarel, I., Brouste, V., Asad-Syed, M. et al. All atypia diagnosed at stereotactic vacuum-assisted breast biopsy do not need surgical excision. Mod Pathol 24, 1198–1206 (2011). https://doi.org/10.1038/modpathol.2011.73
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.73
Keywords
This article is cited by
-
Atypical ductal hyperplasia and the risk of underestimation: tissue sampling method, multifocality, and associated calcification significantly influence the diagnostic upgrade rate based on subsequent surgical specimens
Breast Cancer (2019)
-
Flat epithelial atypia with and without atypical ductal hyperplasia: to re-excise or not. Results of a 5-year prospective study
Virchows Archiv (2012)