Abstract
Carcinomas may arise as a disorder of regeneration, so that a malignant cell may represent a failure to fully attain the characteristics of differentiated tissue. We hypothesized that there is a differential distribution of progenitor cell markers among different histological types of lung cancers, with poorly differentiated tumors being more likely to express progenitor stem cell markers. The study was limited to paraffin-embedded archival material of resected untreated pulmonary carcinomas, including adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell carcinoma. The sections were stained for putative stem cells markers (Musashi-1, Musashi-2, CD34, CD21, KIT, CD133, p63, and OCT-4). Positivity was read as isolated, focal, or diffuse staining. Stem cell markers were detected in all histological types of pulmonary carcinomas. There was a difference in the expression of markers among the histological types. Small cell carcinoma showed diffuse positivity for most of the markers; in contrast to focal or negative staining in other histological groups. An inverse relationship between CD21 and Musashi-1 was observed. No staining for OCT-4 and CD34 was seen in any of the tumor types. Hierarchical clustering based on marker expression separated tumors into two groups, with one group marked by high expression of Musashi-1 and KIT, contained most of the poorly differentiated adenocarcinomas and small cell carcinomas. Therefore, stem cell markers are expressed in lung cancers with different patterns seen for different histological types and degrees of differentiation.
Similar content being viewed by others
Main
Fully differentiated cells arise from stem cells in a process in which they gain markers of differentiation and lose the markers of a stem cell. There is increasing evidence that suggests that cancers arise from maturation-arrest of adult stem cells during regeneration or repair, which results in abnormal differentiation but sustained proliferation.1, 2, 3 If epithelial cancers represent an interruption of differentiation, then it is plausible that cancer cells might gain some markers of differentiation and partially lose expression of stem cell markers.
The lung contains several reserve epithelial cell populations implicated in tissue regeneration after injury, including the basal cell in the main airways, the Clara cells and those of the neuroendocrine body in the terminal bronchial units, and possibly others.4, 5, 6, 7 Lung carcinomas may arise from these reserve epithelial populations.
Pulmonary carcinomas are a heterogeneous group of tumors with varied histological types, including adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell carcinoma. Adenocarcinomas and squamous cell carcinoma have levels of differentiation, whereas large cell carcinoma and small cell carcinomas are thought to represent poorly differentiated carcinomas.
In this study, in an attempt to identify whether progenitor cell markers are expressed in pulmonary carcinomas, the expression of progenitor cell markers across the histological spectrum of pulmonary carcinomas was evaluated by immunohistochemistry.
This study is limited to progenitor cell markers that could be used in archival paraffin-embedded tissue. Some of the markers included are: CD133, a primitive hematopoietic and stem cell marker that shows restricted membranous expression in some epithelial cells and may be a marker of tumor-initiating cells;8, 9 Musashi-1, an RNA-binding protein that seems to be present in cells undergoing asymmetric division and required for the maintenance of stem cell identity;10, 11, 12 Musashi-2, also an RNA-binding protein that belongs to the same family of proteins as Musashi-1;12 and p21, a cyclin-dependent kinase inhibitor of the cell cycle. This protein has been implicated in the maintenance of the quiescence state of hematopoietic stem cells.13 Downregulation of p21 is associated with quiescence and lack of differentiation. p21 is regulated by Musashi-1 through a p53-independent pathway.14
Materials and methods
Surgical specimens from 85 lung tumors, which were surgically resected at Memorial Sloan-Kettering Cancer Center, were reviewed. The tumors were classified according to the World Health Organization (WHO) histological classification of lung tumors.15 Patient data were collected according to the Health Insurance Portability and Accountability Act (HIPAA) regulations. This study was approved by the Memorial Sloan-Kettering Cancer Center Institutional Review Board.
Immunohistochemistry
This study was limited to formalin-fixed paraffin-embedded archival material. Antibodies used were separated into different categories, such as embryonic stem cell marker, OCT-4 (Abcam, Cambridge, MA, USA; dilution 1:50); hematopoietic stem cell markers, such as CD34 (DAKO,USA; dilution 1:1000), KIT (DAKO; dilution 1:800), and CD133 (Miltenyi Biotec, USA; dilution 1:20); markers of asymmetric division, such as Musashi-1 (R&D, USA; dilution 1:800) and Musashi-2 (Protein Tech Group, USA; dilution 1:400); cell cycle differentiation markers, such as p21 (Calbiochem, USA; dilution 1:100); and markers for committed lineage, such as p63 (DAKO; dilution 1:800) for squamous/basal cells, and CC10 and DC-Lamp markers for Clara cell.16
Paraffin sections were de-paraffinized and submitted to antigen retrieval with citrate acid (pH 6) as previously described,16 or with EDTA (pH 8) for the evaluation of p21. Staining was performed on an automated stainer (Ventana Medical System, Tucson, AZ, USA). Histological sections were separately evaluated by two pulmonary pathologists (ALM and NR). Immunostaining for any given antibody was heterogeneous within the histological section. Positivity for a given marker was recorded as diffuse when almost all cells expressed the antigen; focal when isolated groups of positive cells were seen within a histological section; or isolated staining when single cells were positive for the marker.
Correlation of positivity scoring was good (k=0.8) between the two observers for KIT and p21, and Musashi-1 and 2 (k=0.6); and moderate (k=0.4) for p63 and CD133. Discrepancies were resolved by reviewing the slides with re-scoring of the staining pattern at the microscope.
Statistical Analysis
Staining patterns among the groups were compared using the Cochran–Armitage trend test. On a randomly selected subset of the tumors, a second independent reading was obtained and the agreement between the two readings was assessed using Cohen's kappa (poor agreement=less than 0.20; fair agreement=0.20–0.40; moderate agreement=0.40–0.60; good agreement=0.60–0.80; very good agreement=0.80–1.00).
Samples and expression of progenitor cell markers were clustered using hierarchical clustering using Euclidian distance and complete linkage and presented using a heatmap.17, 18
To assess the clustering stability, an R index was calculated by perturbing the data through the introduction of random noise and then re-clustering the perturbed data; this process is repeated multiple times, and the results are compared with the original cluster of unperturbed data. The R index is the proportion of times that a patient pair clusters the same way in the perturbed data sets as in the original data set. In this study, the perturbed data sets were calculated by introducing a small probability (5%) of flipping the binary marker values rather than by the introduction of Gaussian noise.18
Results
The tumors were resected from a patient population comprising of 43 women and 42 men (mean age: 66.8±9.8 years). Tumors included 51 adenocarcinomas, 11 squamous cell carcinomas, 11 large cell carcinomas, and 12 small cell carcinomas (all small cell carcinomas were surgically resected, limited stage tumors). Non-smokers represented 20% of the patients with adenocarcinoma, all other patients were smokers. The distribution by clinical stage of the studied cases was stage IA (41%), IB (13%), IIA (8%), IIB (9%), IIIA (25%), and IIIB (4%). Patients were staged according to AJCC Cancer Staging Manual, 6th edition.19
Immunohistochemical Stains
All four types of pulmonary carcinomas (adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell carcinoma) were negative for OCT-4, an embryonic stem cell marker, and CD34, a marker of hematopoietic stem cells. CD34 exclusively labeled endothelial cells in this study.
Staining of histological sections for KIT/CD117 (stem cell factor receptor) showed no staining for KIT in normal pulmonary epithelial cells, but there was staining in the areas of malignancy. About 14% of adenocarcinomas were diffusely positive and 33% focally positive for KIT. Similarly, 9% of large cell carcinomas were diffusely positive and 36% focally positive for KIT. Small cell carcinomas showed diffuse positivity for KIT in 58% of the cases and 25% showed focal positivity. In contrast, none of the squamous cell carcinomas was diffusely positive for KIT and only 18% were focally positive for this marker. Therefore, KIT showed a differential expression among the four histological types of pulmonary carcinomas (P=0.002).
All adenocarcinomas studied were of mixed subtypes, containing variable combinations of histological patterns, such as acinar, papillary, solid, and bronchioloalveolar carcinoma types. Owing to this histological heterogeneity, the expression of KIT was recorded for each histological pattern of adenocarcinoma. We found that 23% of solid-type adenocarcinoma, a poorly differentiated pattern, was diffusely positive for KIT, whereas the acinar and papillary types showed only 4 and 6% diffuse positivity, respectively. In contrast, areas of bronchioloalveolar carcinoma, a well-differentiated component, showed no positivity for this marker. This suggests that the expression of KIT correlates inversely with histological differentiation in adenocarcinoma.
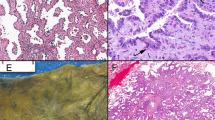
In normal lung tissue, no positivity for CD133 was observed. None of the tumor types showed diffuse expression of CD133; however, some isolated positivity for CD133 was seen in 58% of small cell carcinomas, 63% of large cell lung carcinomas, 19% of adenocarcinomas, and 0% of the squamous cell carcinomas. Positivity for CD133 in small cell carcinomas was restricted to the lumen of rosette-like structures (Figure 1). The differential expression of CD133 was significant among the histological types of pulmonary carcinoma (P=0.008).
Microphotographs showing the expression of Musashi-1 staining. (a) In a normal lung, positive cells are seen interspersed within the ciliated bronchial epithelial cells. (b) Focal positivity is seen in an adenocarcinoma, acinar pattern. (c) Diffuse positivity as is seen in an example of small cell carcinoma. (d) Isolated expression of CD 133 in small cell carcinoma. Note the expression is localized to the apical surfaces of rosette-like formation in these tumors.
In the normal lung, Musashi-1 positivity was observed in scattered cells in terminal bronchioles (Figure 1). Small cell carcinomas (100%) were diffusely positive for Musashi-1. In contrast, diffuse Musashi-1 positivity was noted in only 4% of adenocarcinomas, although 36% showed focal positivity, and 22% isolated positivity for this marker. In large cell carcinomas, 27% of these tumors showed diffuse positivity for Musashi-1, 36% showed focal positivity, and 9% showed isolated positivity. No squamous cell carcinomas show diffuse positivity for this marker; however, 36% of squamous cell carcinomas show focal positivity and 27% show isolated positivity. Therefore, Musashi-1 seems to be differentially expressed among the four groups of lung carcinomas (P<0.001). The differential distribution of Musashi-1 was also seen among the histological subtypes of adenocarcinoma and only solid-type adenocarcinomas showed diffuse positivity for Musashi-1. No diffuse positivity was observed in acinar or papillary types. No positivity, either focal or isolated, was seen for this marker in the well-differentiated bronchioloalveolar carcinomas.
In the normal lung, expression of Musashi-2 is seen in the terminal bronchioles and peritumoral reactive type II pneumocytes (data not shown). Similar to Musashi-1, 100% small cell carcinomas showed diffuse positivity for Musashi-2. Diffuse positivity for Musashi-2 was also seen in 25% of adenocarcinomas, 36% of large cell lung cancers, and 45% of squamous cell carcinoma. The differential expression of Musashi-2 was statistically significant among the histological groups (P=0.008).
An inverse pattern of Musashi-1 and p21 expression was observed in pulmonary carcinomas, with a correlation index of r=–0.25. There is no significant difference in the expression of p21 among the four histological types of pulmonary carcinoma (P=0.37).
There was no co-localization of staining for different markers to the same group of cells in case of isolated or focal positivity. Therefore, the expression of progenitor cell markers was heterogeneous within the histological sections.
Expression of lineage specific markers, such as CC10 and DC-Lamp (which are expressed on Clara cells), was restricted to adenocarcinomas. Diffuse positivity for these two markers was seen in 30% of bronchioloalveolar carcinomas. Other subtypes of adenocarcinomas show focal or isolated staining for these markers, similar to what has been shown previously.16 By contrast, p63, which is a protein associated with epithelial stratification and squamous differentiation,20, 21 was expressed diffusely in 14% of adenocarcinomas, 27% of large cell carcinomas, and 0% of the small cell carcinomas studied. As expected, 100% of the squamous cell carcinomas showed diffuse and strong positivity for this marker. The differential expression of p63 among the histological groups of pulmonary carcinoma was statistically significant (P<0.001).
On examining the expression of p63 in the subtypes of adenocarcinoma, we observed diffuse positivity in the bronchioloalveolar carcinomas, acinar, and papillary subtypes, but no diffuse positivity was observed in the solid-type adenocarcinomas.
There was a differential expression of progenitor cell markers among the different subtypes of adenocarcinoma. Poorly differentiated types expressed musahi-1 and KIT, whereas well-differentiated types stained preferentially for p63 (Figure 2).
Percentage of diffuse expression of progenitor cell markers p63, KIT and Musashi-1 in adenocarcinoma subtypes. Note that p63 a marker associated with cell stratification is seen in well-differentiated subtypes of adenocarcinomas, whereas diffuse expression of musashi-1 is seem only in solid-type pattern.
Hierarchical Clustering
A clustering algorithm was used to analyze the distribution of progenitor cell markers on the basis of the immunohistochemical data among the four histological types of pulmonary carcinoma.
The resulting dendrogram (Figure 3) suggests a separation of expression profile into two groups: the first group being characterized by the expression of Musashi-1, KIT, and CD133, and the second by the expression of Musashi-2 and p63.
Dendrogram showing the expression profile of markers. Each row indicates a patient and each column the expression profile. In a color scale, lighter color indicates diffuse expression. Histological diagnosis is indicated in the bar (navy blue: adenocarcinoma; baby blue: large cell carcinoma; yellow: squamous cell carcinoma; pink: small cell carcinoma). The column dendrogram indicates clustering of markers expression and the row dendrogram indicates clustering of histological type. c-kit (KIT), Mush-1 (Musashi-1), and Mush-2 (Musashi-2).
The R index for the hierarchical clustering was 0.78, suggesting a good level of reproducibility of the clusters produced in this analysis.
This analysis also indicates that the tumors can be clustered into groups on the basis of the expression of markers. Although each histological subtype was represented within each cluster, their distribution was significantly different (P<0.001) with one group characterized by high expression of KIT, CD133, and Musashi-1 contained most of the small cell carcinomas, whereas the other group contained most of the squamous cells carcinomas.
Discussion
If carcinomas arise as a result of an arrest in maturation of stem cells, a malignant cell represents both a failure to fully attain the characteristics of a differentiated tissue and also to shed the characteristics of a stem cell. If so, cancer cells would be expected to share the characteristics of both stem and differentiated cells, with poorly differentiated tumors being more likely to express stem cell markers. Our results indicate that there is a differential distribution of progenitor cell markers among the different histological types of lung cancer and that poorly differentiated tumors have the highest expression of these markers.
Small cell carcinomas are usually aggressive neoplasm with high metastatic potential and recurrence rate. Our results suggest that small cell carcinomas express the highest number of progenitor cell markers as compared to the other histological types of lung carcinoma. Small cell carcinomas demonstrate diffuse expression of Musashi-1, Musashi-2, and KIT, whereas focal or isolated positivity was seen in most of other types of pulmonary carcinoma. This finding is in line with previous study in which other authors also found similarities in the expression of stem cell markers and small cell carcinoma.22
In adenocarcinomas, histological heterogenous tumors composed of a mixture of several histological patterns, diffuse expression of the progenitor cell markers, Musashi-1 and KIT, is seen in a low percentage of tumors; however, it is seen most frequently in the solid type that is considered to be poorly differentiated. Adenocarcinomas with a predominant solid-type component are associated with poor prognosis.23, 24 In contrast, bronchioloalveolar carcinoma, a well-differentiated type that is associated with excellent prognosis,25, 26 expressed markers that are associated with differentiation, such as DC-Lamp, CC10 (Clara cells), and p63. There was no expression of Musashi-1 or KIT in this adenocarcinoma type. Interestingly, the expression of Clara cell markers seems to be limited to well-differentiated adenocarcinomas. This suggests that Clara cells may represent a more committed cell lineage rather than a true stem cell in the human lung. In mice, in which the exploration of the terminal bronchial niche can be performed experimentally, a pluripotent variant of ‘Clara cell’ has been identified in the bronchioloalveolar duct junction.27
It has long been held that the lung contains multiple different progenitor cells that are thought to be the cell of origin of the different histological types of pulmonary carcinoma. For instance, squamous cell carcinomas are thought to arise from a squamous metaplasia-dysplasia sequence of the central airway basal cells.15, 28 By contrast, adenocarcinomas, more commonly a peripheral tumor, are felt to arise from progenitor cells in the terminal respiratory unit (TRU).29 There is no evidence of a precursor cell for small cell carcinoma. Our results, however, suggest a great overlap of progenitor/stem cell markers among the different histological types of lung carcinomas. This may be an indication of a common cell of origin or a common pathway of cell differentiation. In fact, dual differentiation, such as that seen in adenosquamous carcinomas or combined small cell/non-small cell carcinomas, is a well-recognized phenomenon in pulmonary pathology.15
This study was based on immunohistochemistry of archival paraffin-embedded tissue, which limits our ability to include other markers associated with stem cells, such as those used in the detection of antigens by flow cytometry of dissociated tumor cells or cell lines.30, 31, 32, 33, 34 Immunohistochemistry may have also limited our ability to detect antigens that are expressed in a small number of cells. This may be particularly relevant to the low detection of antigen, such as CD133 and CD34. The latter has been shown to be present in a small fraction of malignant epithelial cells,2, 8, 9 but was not found in this study. The absence of staining in pulmonary carcinomas for OCT-4, an embryonic stem cell marker, is confirmatory.35, 36 The OCT-4 staining is positive in germ-cell tumors, such as seminomas and embryonal carcinomas, but not in somatic tumors.
Musashi-1 is an RNA-binding protein implicated in asymmetric cell division and is considered to be a marker for neural and intestinal stem cells.37, 38, 39, 40 Musashi-1 downregulates the expression of Numb protein,11 which in turn regulates Notch receptor activity.41 As a protein implicated in asymmetric cell division, Musashi-1 also downregulates the expression of p21 by a mechanism independent of p53.14 p21 is associated with maintenance of quiescent state in stem cells.13, 14 Upregulation of p21 and downregulation of Notch are associated with cell differentiation. Our finding that there was an inverse correlation between the expression of p21 and Musashi-1 in pulmonary carcinomas is consistent with these observations.
Some in vitro studies have suggested that stem cells lose expression of stem cell markers as they differentiate.42, 43 In our study, we observed that some markers were expressed focally in tumor sections, but there was no co-expression of markers in the same group of cells. This finding may suggest a sequential loss of marker expression during the maturation process, or that within the same tumor there might be different clones of progenitor cells in different stages of maturation depending on how far through the differentiation process the progenitor had proceeded before the process was interrupted. Alternatively, it should be noted that it is likely that stem cells are recruited into cancers during the ongoing process of carcinogenesis and these recruited cells could give rise to focally positive areas of stem cell markers. A similar clustering of bone marrow-derived cells was observed in tumors from patients who had received a bone marrow transplant from a sex-mismatched donor.44
In summary, this study demonstrates that progenitor cell markers are differentially expressed in histological types of pulmonary carcinomas in a manner that it is associated with tumor differentiation. In addition, the Musashi/Notch pathway seems to have a role in small cell carcinoma. A larger study designed to investigate the association of progenitor cell markers expression, prognostic markers, or therapeutic intervention as an end point may be required to better address this question.
References
Borczuk AC, Gorenstein L, Walter KL, et al. Non-small-cell lung cancer molecular signatures recapitulate lung developmental pathways. Am J Pathol 2003;163:1949–1960.
Piscaglia AC, Shupe TD, Petersen BE, et al. Stem cells, cancer, liver, and liver cancer stem cells: finding a way out of the labyrinth. Curr Cancer Drug Targets 2007;7:582–590.
Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–111.
Coraux C, Roux J, Jolly T, et al. Epithelial cell-extracellular matrix interactions and stem cells in airway epithelial regeneration. Proc Am Thorac Soc 2008;5:689–694.
Franks TJ, Colby TV, Travis WD, et al. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc Am Thorac Soc 2008;5:763–766.
Kotton DN, Fine A . Lung stem cells. Cell Tissue Res 2008;331:145–156.
Weiss DJ, Kolls JK, Ortiz LA, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2008;5:637–667.
Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2008;15:504–514.
Mizrak D, Brittan M, Alison MR . CD133: molecule of the moment. J Pathol 2008;214:3–9.
Nakamura M, Okano H, Blendy JA, et al. Musashi, a neural RNA-binding protein required for Drosophila adult external sensory organ development. Neuron 1994;13:67–81.
Okano H, Imai T, Okabe M . Musashi: a translational regulator of cell fate. J Cell Sci 2002;115:1355–1359.
Siddall NA, McLaughlin EA, Marriner NL, et al. The RNA-binding protein Musashi is required intrinsically to maintain stem cell identity. Proc Natl Acad Sci USA 2006;103:8402–8407.
Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 2000;287:1804–1808.
Battelli C, Nikopoulos GN, Mitchell JG, et al. The RNA-binding protein Musashi-1 regulates neural development through the translational repression of p21WAF-1. Mol Cell Neurosci 2006;31:85–96.
Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. IARC press: Lyon, France, 2004.
Zhu LC, Yim J, Chiriboga L, et al. DC-LAMP stains pulmonary adenocarcinoma with bronchiolar Clara cell differentiation. Hum Pathol 2007;38:260–268.
Jarnagin WR, Klimstra DS, Hezel M, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol 2006;24:1152–1160.
McShane LM, Radmacher MD, Freidlin B, et al. Methods for assessing reproducibility of clustering patterns observed in analyses of microarray data. Bioinformatics 2002;18:1462–1469.
Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Maunal. eds. Springer-Verlag: New York. 6th edn 2002.
Koster MI, Kim S, Mills AA, et al. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 2004;18:126–131.
Wang BY, Gil J, Kaufman D, et al. P63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol 2002;33:921–926.
Koch LK, Zhou H, Ellinger J, et al. Stem cell marker expression in small cell lung carcinoma and developing lung tissue. Hum Pathol 2008;39:1597–1605.
Barletta JA, Yeap BY, Chirieac LR . Prognostic significance of grading in lung adenocarcinoma. Cancer 2010;116:659–669.
Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008;32:810–827.
Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844–2852.
Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol 2007;20:233–241.
Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835.
Auerbach O, Stout AP, Hammond EC, et al. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med 1961;265:253–267.
Tanaka H, Yanagisawa K, Shinjo K, et al. Lineage-specific dependency of lung adenocarcinomas on the lung development regulator TTF-1. Cancer Res 2007;67:6007–6011.
Eckfeldt CE, Mendenhall EM, Verfaillie CM . The molecular repertoire of the ‘almighty’ stem cell. Nat Rev Mol Cell Biol 2005;6:726–737.
Ho MM, Ng AV, Lam S, et al. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res 2007;67:4827–4833.
Laurson J, Selden C, Hodgson HJ . Hepatocyte progenitors in man and in rodents—multiple pathways, multiple candidates. Int J Exp Pathol 2005;86:1–18.
Sutherland DR, Keeney M . Re: selection of stem cells by using antibodies that target different CD34 epitopes yields different patterns of T-cell differentiation. Stem Cells 2007;25:2385–2386.
Villadsen R . In search of a stem cell hierarchy in the human breast and its relevance to breast cancer evolution. APMIS 2005;113:903–921.
Cantz T, Key G, Bleidissel M, et al. Absence of OCT4 expression in somatic tumor cell lines. Stem Cells 2008;26:692–697.
Mueller T, Luetzkendorf J, Nerger K, et al. Analysis of OCT4 expression in an extended panel of human tumor cell lines from multiple entities and in human mesenchymal stem cells. Cell Mol Life Sci 2009;66:495–503.
Kaneko Y, Sakakibara S, Imai T, et al. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci 2000;22:139–153.
Nishimura S, Wakabayashi N, Toyoda K, et al. Expression of Musashi-1 in human normal colon crypt cells: a possible stem cell marker of human colon epithelium. Dig Dis Sci 2003;48:1523–1529.
Potten CS, Booth C, Tudor GL, et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 2003;71:28–41.
Sakakibara S, Imai T, Hamaguchi K, et al. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol 1996;176:230–242.
Bolos V, Blanco M, Medina V, et al. Notch signalling in cancer stem cells. Clin Transl Oncol 2009;11:11–19.
Herrera MB, Bruno S, Buttiglieri S, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 2006;24:2840–2850.
Shin S, Mitalipova M, Noggle S, et al. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells 2006;24:125–138.
Avital I, Moreira AL, Klimstra DS, et al. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells 2007;25:2903–2909.
Acknowledgements
We thank Dr Robert Maki and Dr Robert Soslow for their suggestions and critical review of this paper; Cymra McBean for help in preparing the article; Daniel Friedenberg for help in the maintenance of database; and Marina Asher and Irina Linkov for technical assistance with immunohistochemical assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Moreira, A., Gonen, M., Rekhtman, N. et al. Progenitor stem cell marker expression by pulmonary carcinomas. Mod Pathol 23, 889–895 (2010). https://doi.org/10.1038/modpathol.2010.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.68
Keywords
This article is cited by
-
A potentially functional polymorphism in ABCG2 predicts clinical outcome of non-small cell lung cancer in a Chinese population
The Pharmacogenomics Journal (2017)
-
Thymus neuroendocrine tumors with CTNNB1 gene mutations, disarrayed ß-catenin expression, and dual intra-tumor Ki-67 labeling index compartmentalization challenge the concept of secondary high-grade neuroendocrine tumor: a paradigm shift
Virchows Archiv (2017)
-
Pluripotency transcription factors in lung cancer—a review
Tumor Biology (2016)
-
Musashi-1 Expression is a Prognostic Factor in Ovarian Adenocarcinoma and Correlates with ALDH-1 Expression
Pathology & Oncology Research (2015)
-
Expression of seven stem-cell-associated markers in human airway biopsy specimens obtained via fiberoptic bronchoscopy
Journal of Experimental & Clinical Cancer Research (2013)