Abstract
The purpose of this study was to confirm that the grades of lymph vessel tumor emboli in biopsy specimens obtained before neoadjuvant therapy and in the surgical specimens obtained after neoadjuvant therapy according to the grading system we devised are significant histological outcome predictor for invasive ductal carcinoma (IDC) patients who received neoadjuvant therapy. The subjects of this study were the 318 consecutive IDC patients who had received neoadjuvant therapy in our institution. The lymph vessel tumor embolus grades in the biopsy specimens and in the surgical specimens were significantly associated with the increases in mean number of nodal metastases. Multivariate analyses with well-known prognostic factors and p53 expression in tumor-stromal fibroblasts clearly showed that the lymph vessel tumor embolus grade based on the biopsy specimens and based on the surgical specimens significantly increased the hazard rates for tumor recurrence and tumor-related death in all the IDC patients as a whole, in the IDC patients who did not have nodal metastasis, and in the IDC patients who had nodal metastasis, and the outcome-predictive power of the lymph vessel tumor embolus grades based on the surgical specimens was superior to that of the lymph vessel tumor embolus grades based on the biopsy specimens. The grades in the grading system for lymph vessel tumor emboli were significantly associated with nodal metastasis, and the histological grading system is an excellent system for accurately predicting the outcome of patients with IDC of the breast who have received neoadjuvant therapy.
Similar content being viewed by others
Main
Lymphatic invasion in breast cancer patients with invasive ductal carcinoma (IDC) has been reported to have prognostic significance.1, 2, 3, 4, 5 We have already reported that the grading system for lymph vessel tumor emboli that we devised is a very useful histological grading system for accurately predicting the outcome of patients with IDC who did not receive neoadjuvant therapy, and that the grading system can be used to classify IDC patients with lymph vessel invasion into a low-, intermediate-, and high-risk groups for outcome.6 Furthermore, we have recently reported finding that the lymph vessel tumor embolus grades based on the biopsy specimens and based on the surgical specimens are also an important outcome-predictive factor for IDC patients who received neoadjuvant chemotherapy and had nodal metastasis.7
The purpose of this study was to confirm that the grading system for lymph vessel tumor embolus is a significant outcome predictor for IDC patients who received neoadjuvant therapy according to nodal status, by multivariate analysis with well-known clinicopathological factors and p53 protein expression in tumor-stromal fibroblasts in IDCs.8 p53 protein expression in tumor-stromal fibroblasts, but not in tumor cells, in IDCs has been recently demonstrated to be a very important outcome predictor for IDC patients who received neoadjuvant therapy.8 The results of this study clearly showed that the grading system for lymph vessel tumor emboli is an excellent histological outcome predictive of the histological grading system for IDC patients who received neoadjuvant therapy independent of nodal metastasis.
Materials and methods
Cases
The subjects of this study were the 318 consecutive patients with IDC of the breast who received neoadjuvant therapy and were surgically treated at the National Cancer Center Hospital between January 2000 and December 2005 (the same series of patients as in an earlier study we conducted8 and 88 patients of the 318 patients in this series were included among the subjects of our previous study7). Their IDCs were diagnosed preoperatively by needle biopsy, aspiration cytology, mammography, or ultrasonography. Clinical information was obtained from the patients’ medical records after complete histological examination of all IDCs. All patients were Japanese women, and they ranged in age from 23 to 77 years old (median, 55 years). All had a solitary lesion; 127 patients were premenopausal, and 191 were postmenopausal. Partial mastectomy had been performed in 152, and modified radical mastectomy in 166. Level I and Level II axillary lymph node dissection had been performed in all patients, and Level III axillary lymph node dissection had been performed in some of the IDC patients.
Of the 318 subjects, 37 (12%) had shown a pathological complete response to neoadjuvant therapy (34, no residual tumor and no nodal metastasis; 3, residual ductal carcinoma in situ and no nodal metastasis).
The neoadjuvant therapy consisted of chemotherapy in 235 patients, endocrine therapy in 43 patients, and chemoendocrine therapy in 3 patients, and 214 out of the 281 patients who had received neoadjuvant therapy had also received adjuvant therapy, which consisted of chemotherapy in 47 patients, endocrine therapy in 116 patients, and chemoendocrine therapy in 51 patients. The chemotherapy regimens used were anthracycline-based with or without taxane and non-anthracycline-based, and the endocrine therapy regimens consisted of tamoxifen with or without a gonadotropin-releasing-hormone agonist, tamoxifen with or without an aromatase inhibitor, an aromatase inhibitor alone, or a gonadotropin-releasing-hormone agonist alone. There were no cases of inflammatory breast cancer in this series. All tumors were classified according to the pathological UICC-TNM (pTNM) classification system.9 The protocol of this study (20–112) was reviewed by the institutional review board of the National Cancer Center, and all patients provided written informed consent.
For the pathological examination, biopsy specimens obtained before neoadjuvant therapy and surgically resected specimens obtained after neoadjuvant therapy were fixed in 10% formalin and subsequently examined. The size and gross appearance of the surgically resected tumor specimens were recorded as the residual invasive tumor size. The tumor size of the surgically resected specimens was confirmed by comparison with the tumor size on histological slides; if more than one invasive focus was present, the size of the largest invasive focus was recorded as the residual invasive tumor size in this study.
Histological Examination
Serial sections of the biopsy specimens obtained before neoadjuvant chemotherapy and of the tumor area in the surgically resected specimens obtained after neoadjuvant therapy were cut from paraffin wax blocks. One section of each biopsy specimen and surgical specimen was stained with hematoxylin and eosin and examined histologically to confirm the diagnosis, and another section was subjected to immunohistochemistry. The following eight histological features of the primary-invasive tumors were evaluated in the biopsy specimens obtained before neoadjuvant therapy and the surgical specimens obtained after neoadjuvant therapy: (1) residual tumor size (no residual tumor or residual ductal carcinoma in situ, residual tumor ≤20 mm, >20 to ≤50 mm, >50 mm), (2) histological grade (1, 2, 3),10 (3) tumor necrosis (absent, present),11 (4) fibrotic focus (biopsy specimen: absent, present; surgical specimen: absent, fibrotic focus diameter <8 mm, fibrotic focus diameter >8 mm),12, 13 (5) blood vessel invasion (absent, present), (6) adipose tissue invasion (absent, present), (7) skin invasion (absent, present), and (8) muscle invasion (absent, present). We also evaluated the outcome-predictive power of Fisher's neoadjuvant therapy effect classification for surgical specimens obtained after neoadjuvant therapy.14, 15
Grading System for Lymph Vessel Tumor Emboli in IDCs
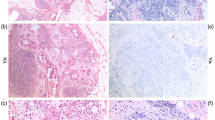
We have already stated the histological criteria of the grading system for lymph vessel tumor emboli in our previous study.6, 7 Briefly, we first examined all slides of IDCs that contained both tumor areas and nontumor areas to identify lymph vessel tumor emboli. Next, we selected the lymph vessel tumor emboli, for example, large lymph vessel tumor emboli far from the stroma-invasive tumor margin to examine for the presence and number of mitotic figures and apoptotic figures of lymph vessel tumor emboli in an IDC (Figures 1a and c). We classified all IDCs into the following four grades according to the number of mitotic and apoptotic figures in tumor cells in lymph vessels in one per high-power field: grade 0, no lymph vessel invasion; grade 1, absence of no mitotic and or apoptotic figures, or presence of any number of mitotic figures and absence of but no apoptotic figures, or absence of mitotic figure and presence of any number of apoptotic figures but no mitotic figures; grade 2, 1–4 mitotic figures and ≥1 apoptotic figures, or ≥1 mitotic figures and 1–6 apoptotic figures; and grade 3, >4 mitotic figures and >6 apoptotic figures. We selected the lymph vessel tumor emboli in which to count the mitotic figures and apoptotic figures and then recorded the numbers of mitotic figures and apoptotic figures among the tumor cells making up the lymph vessel tumor emboli of the IDC in the high-power field that contained the largest number of mitotic figures, and/or the largest number of apoptotic figures were recorded as the number of mitotic figures and apoptotic figure in the lymph vessel tumor emboli of the IDC. For the biopsy specimens, we examined the presence or absence of lymph vessel tumor embolus or lymph vessel tumor emboli; when lymph vessel tumor embolus or lymph vessel tumor emboli were observed in the biopsy specimen, an assessment similar to that described above was performed. Some IDCs contained large lymph vessel tumor emboli, especially in IDCs containing a grade 2 or 3 lymph vessel tumor emboli, and it was difficult to determine whether they were true lymph vessel tumor emboli or a non-IDC component by hematoxylin and eosin staining alone. We therefore performed immunohistochemical staining with D2-40 antibody (monoclonal mouse antibody, Signet, Dedham, MA, USA, 1:200) to confirm that the lymph vessel tumor emboli identified by hematoxylin and eosin staining in some of the IDCs with grade 2 or 3 lymph vessel tumor emboli were true tumor emboli (Figure 1b). The D2-40 antibody was generated against an O-linked sialoglycoprotein having a molecular weight of 40 kDa and had been shown to be a selective marker of the lymphatic endothelium.16, 17
(a) Two lymph vessel tumor emboli are observed. (b) Vessel walls positive for D2-40 around two lymph vessel tumor emboli can be seen (arrows). One small lymph vessel is also positive for D2-40 (arrow), and one artery is negative for D2-40 (arrowhead). (c) Several apoptotic bodies and apoptotic tumor cells are observed (arrowheads), and there are five mitotic tumor cells (arrows) in tumor embolus in the lymph vessel. Apoptotic bodies are small, variously shaped pyknotic bodies that resemble sesame seeds, and apoptotic tumor cells were identified as tumor cells that contained eosinophilic or amphophilic cytoplasm and an irregularly shaped pyknotic nucleus. (d) Three lymph vessel tumor emboli with an Allred score of 8 for p53 can be seen. More than 90% of the tumor cells comprising of the lymph vessel tumor emboli show an intense nuclear staining for p53.
Immunohistochemistry
Immunohistochemical staining for estrogen receptors, progesterone receptors, p53, HER2 products, and D2-40 was performed with an autoimmunostainer (Optimax Plus: BioGenex, San Ramon, CA, USA). The antigen retrieval device used for the Optimax Plus was an autoclave, and each specimen was immersed in citrate buffer and incubated at 121°C for 10 min. Immunoperoxidase staining was performed by using a labeled streptavidin biotin staining kit (BioGenex) according to the manufacturer's instructions. The antibodies used were an anti-estrogen receptor mouse monoclonal antibody (mAb), ER88 (BioGenex), an anti-progesterone receptor mAb, PR88 (BioGenex), and an anti-HER2 mAb, CB11 (BioGnex), and a p53 mAb, DO7 (Dako, Glostrup, Denmark). ER88, PR88, and CB11 were already diluted, and DO7 was applied at a 1:100 dilution. After immunostaining, the sections were counterstained with hematoxylin. Sections of IDCs positive for estrogen receptor, progesterone receptor, p53, HER2, and D2-40 were used each time as positive controls. As for a negative control, the primary antibody was replaced with normal mouse immunoglobulin.
Slides immunostained for estrogen receptor, progesterone receptor, and p53 in stroma-invasive tumor cells, and for p53 in tumor-stromal fibroblasts were scored by the Allred scoring system as previously described.8, 18, 19, 20, 21, 22 The highest intensity score, not the average intensity score, for nuclear expression of p53 was assigned for in tumor-stromal fibroblasts, and the highest p53 nuclear expression proportion score and intensity score were then to be evaluated in one high-power field ( × 40 objective and × 10 ocular).8 The Allred scores for estrogen receptor, progesterone receptor, and p53 expression in stroma-invasive tumor cells and tumor-stromal fibroblasts were classified into the following three categories: (1) Allred score for estrogen receptor in stroma-invasive tumor cells, 0 or 2, 3–6, and 7 or 8; (2) Allred score for progesterone receptor in stroma-invasive tumor cells, 0 or 2, 3–6, and 7 or 8; (3) Allred scores for p53 in stroma-invasive tumor cells, 0 or 2 or 3, 4–6, and 7 or 8; and (4) Allred scores for p53 in tumor-stromal fibroblasts, 0 or 2, 3, and 4–8. HER2 expression in stroma-invasive tumor cells was classified into the three categories: 0 or 1, 2, and 3.23 We also assigned Allred scores for estrogen receptor, progesterone receptor, and p53 (Figure 1d), and HER2 category in lymph vessel tumor emboli by the similar manner as in stroma-invasive tumor cells in 26 of the 82 IDCs with lymph vessel invasion. We were unable to assign Allred scores for estrogen receptor, progesterone receptor, and p53, and HER2 category in the other IDCs with lymph vessel invasion, because immunohistochemistry for these was performed in tumor tissue sections that did not containing an lymph vessel tumor embolus.
One author (TH) assessed all the immunohistochemical parameters, and one of four other authors (NT, HT, TS, or YS) reviewed the immunohistochemical parameters to confirm the IDC immunohistochemical characteristics recorded by TH. Discordant results were reevaluated jointly to reach until a consensus was reached. The histological examination and immunohistochemical examination were performed without knowledge of the patient's outcome.
Patient Outcome and Statistical Analysis
Survival was evaluated by follow-up for a median period of 62 months (range: 38–105 months) until February 2009. As of the end of February 2009, 191 of the 281 patients were alive and well, 90 had developed tumor recurrence, and 53 had died of their disease. The measurements of tumor-recurrence-free survival, and overall survival started at the time of surgery. Tumor relapse was considered to have occurred whenever there was evidence of metastasis.
Multiple regression analysis was used to perform the statistical analyses for associations between lymph vessel tumor embolus grade and number of lymph node metastases, and the correlation analyses were performed by the correlation statistics of Cochran–Mantel–Haenszel statistics. We analyzed the outcome-predictive power for tumor recurrence and tumor-related death by the univariate and multivariate analyses using the Cox proportional hazard regression model. The factors analyzed were the mentioned eight factors, age (≤39, >39 years), type of neoadjuvant therapy (endocrine therapy, chemotherapy and chemoendocrine therapy), adjuvant therapy (no, yes), and the factors that were significantly associated with outcome in the univariate analyses were then entered together into the multivariate analyses according to nodal status. As the eight factors were examined using both biopsy specimens obtained before neoadjuvant therapy and surgical specimens obtained after neoadjuvant chemotherapy, to accurately assess the prognostic value of each of these factors in multivariate analyses, their mutual influence on outcome was avoided by conducting separate analyses of the prognostic predictive power of the findings in the biopsy specimens obtained before neoadjuvant therapy and the surgical specimens obtained after neoadjuvant therapy (model 1, factors examined based on biopsy specimens obtained before neoadjuvant therapy; model 2, factors examined based on surgical specimens obtained after neoadjuvant therapy). The case-wise and step-down method was applied until all the remaining factors were significant at a P-value below 0.05. As there were fewer than 10 tumor deaths among the patients who did not have nodal metastasis, we were unable to perform multivariate analyses for tumor death in this groups. Survival curves were drawn by the Kaplan–Meier method. All analyses were performed with Statistica/Windows software (StatSoft, Tulsa, OK, USA).
Results
Associations Between the Lymph Vessel Tumor Embolus Grades and Factors
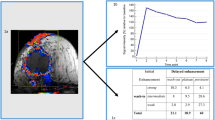
Although the lymph vessel tumor embolus grades based on the biopsy specimens and based on the surgical specimens were significantly associated with the increases in mean number of nodal metastases (Figure 2), the value of β for the correlation between lymph vessel tumor embolus grades in the surgical specimens and mean number of nodal metastases was higher than between the lymph vessel tumor embolus grades in the biopsy specimens and the mean number of nodal metastases.
Graphs showing associations between mean nodal metastasis values and the grades of lymph vessel tumor emboli in the biopsy specimens (a) and in the surgical specimens (b). The mean number of nodal metastases increases significantly with the grade of lymph vessel tumor emboli in the biopsy specimens and in the surgical specimens.
The results of the univariate analyses showed that the lymph vessel tumor embolus grades assessed in the surgical specimens were significantly associated with the Allred scores for p53 in stroma-invasive tumor cells and in tumor-stromal fibroblasts assessed in the surgical specimens (Table 1) and that they were also significantly inversely associated with Allred score for estrogen receptor in stroma-invasive tumor cells assessed in the surgical specimens in the univariate analyses (data not shown). There was no significant association between lymph vessel tumor embolus grades in the surgical specimens and progesterone receptor in stroma-invasive tumor cells in the surgical specimens, and between lymph vessel tumor embolus grades in the surgical specimens and HER2 category in tumor cells in the surgical specimens (data not shown). The Allred scores for p53 in lymph vessel tumor emboli assessed in the surgical specimens were significantly associated with the grades of the lymph vessel tumor emboli assessed in the surgical specimens (Table 1), but there were no significant associations between the Allred scores for estrogen receptor or progesterone receptor in lymph vessel tumor emboli, or the HER2 categories in the lymph vessel tumor emboli and grades of lymph vessel tumor embolus assessed in the surgical specimens (data not shown).
In our previous study, although the multivariate analysis clearly showed that negative nodal status and HER2 category 3 in tumor cells were significantly associated with pathological complete response,8 the lymph vessel tumor embolus grades based on the biopsy specimens were not significantly associated with pathological complete response in the univariate analysis (data not shown).
Factors Significantly Associated with Outcome
The univariate analyses of all of the cases as a whole showed that the lymph vessel tumor embolus grades in the biopsy specimens (Figures 3a and b) and the surgical specimens (Figures 3c and d) were significantly associated with tumor recurrence and tumor-related death (Table 2). In the multivariate analyses using model 1, UICC pTNM-pN1, N2, and N3 categories significantly increased the hazard rates for tumor recurrence, and UICC pTNM-pN2 and N3 also significantly increased the hazard rates for tumor-related death (data not shown). The Allred score of 3 for p53 in tumor-stromal fibroblasts and Allred scores of 4–8 for p53 in tumor-stromal fibroblasts significantly increased the hazard rates for tumor recurrence, and lymph vessel tumor embolus grades 2 and 3 also significantly increased the hazard rate for tumor recurrence in the multivariate analyses (data not shown). Allred scores of 7 or 8 for estrogen receptors in tumor cells significantly decreased the hazard rate for tumor-related death, and HER2 category 3 in tumor cells significantly increased the hazard rate for tumor-related death in the multivariate analyses (data not shown). When model 2 was used, lymph vessel tumor embolus grade 2 and lymph vessel tumor embolus grade 3 significantly increased the hazard rates for tumor recurrence and tumor-related death in the multivariate analyses (data not shown). The Allred score of 3 in tumor-stromal fibroblasts, Allred scores of 4–8 for p53 in tumor-stromal fibroblasts, and histological grade 3 significantly increased the hazard rates for tumor recurrence, and the Allred scores of 4–8 in tumor-stromal fibroblasts and histological grade 3 also significantly increased the hazard rate for tumor-related death in the multivariate analyses (data not shown). Residual invasive tumor size >50 mm significantly increased the hazard rate for tumor recurrence and the presence of skin invasion significantly increased the hazard rate for tumor-related death in the multivariate analyses (data not shown).
Disease-free survival curves (a and c) and overall survival curves (b and d) of all of the invasive ductal carcinoma (IDC) patients who received neoadjuvant therapy as a whole. (a and b) The disease-free survival time and the overall survival time of the IDC patients classified by grade of lymph vessel tumor emboli in the biopsy specimens obtained before neoadjuvant therapy become significantly shorter as the grades of lymph vessel tumor emboli increased. (c and d) The disease-free survival time and the overall survival time of the IDC patients classified by the grade of lymph vessel tumor emboli in the surgical specimens become significantly shorter as the grades of lymph vessel tumor emboli increased. None of the IDC patients had grade 1 lymph vessel tumor emboli show tumor-related death.
In the group of IDC patients without nodal metastasis, the univariate analyses showed that the lymph vessel tumor embolus grades in the biopsy specimens were significantly associated with tumor recurrence, but not with tumor-related death, and that the lymph vessel tumor embolus grades in the surgical specimens were significantly associated with both tumor recurrence and tumor-related death (Table 2). In the multivariate analyses, lymph vessel tumor embolus grades 1 and 2 in the biopsy specimens, Allred scores of 4–8 for p53 in tumor-stromal fibroblasts in the biopsy specimens, and age ≤38 years significantly increased the hazard rates for tumor recurrence in the multivariate analyses (Table 3, model 1), and lymph vessel tumor embolus grade 2 in the surgical specimens, Allred score of 3 for p53 in tumor-stromal fibroblasts in the surgical specimens, and Allred scores of 4–8 for p53 in tumor-stromal fibroblasts in the surgical specimens significantly increased the hazard rates for tumor recurrence in the multivariate analysis (Table 3, model 2).
In the group of IDC patients with nodal metastasis, the univariate analyses showed that the lymph vessel tumor embolus grades in the biopsy specimens and the surgical specimens were significantly associated with tumor recurrence and tumor-related death (Table 2). In the multivariate analyses of model 1, lymph vessel tumor embolus grades 2 and 3, an Allred score of 3 for p53 in tumor-stromal fibroblasts, and Allred scores of 4–8 for p53 in tumor-stromal fibroblasts significantly increased the hazard rate for tumor recurrence, and lymph vessel tumor embolus grade 1 significantly increased the hazard rate for tumor-related death (Table 4). The Allred scores of 7 or 8 for estrogen receptors in tumor cells significantly decreased the hazard rate for tumor recurrence, and Allred scores of 3–6 for estrogen receptors in tumor cells significantly decreased the hazard rate for tumor death in the multivariate analyses (Table 4). UICC pN3 category significantly increased the hazard rate for tumor recurrence, and HER2 category 3 in tumor cells, histological grade 3, and absence of adjuvant therapy significantly increased the hazard rates for tumor-related death in the multivariate analyses (Table 4). In model 2, lymph vessel tumor embolus grade 2, lymph vessel tumor embolus grade 3, Allred scores of 4–8 for p53 in tumor-stromal fibroblasts, and histological grade 3 significantly increased the hazard rates for tumor recurrence and tumor-related death in the multivariate analyses (Table 4). Residual invasive tumor size >50 mm and Allred scores of 7 or 8 for p53 in tumor cells significantly increased the hazard rates for tumor recurrence, and the presence of skin invasion significantly increased the hazard rate for tumor-related death in the multivariate analysis (Table 4).
Discussion
The results of this study clearly showed significant associations between increases in grade of lymph vessel tumor embolus assessed in the biopsy specimens and surgical specimens and the number of nodal metastases. We have also found a significant association between grade of lymph vessel tumor embolus and number of nodal metastases in a different no-neoadjuvant therapy IDC group in another study.6 Thus, the grading system for lymph vessel tumor embolus can be concluded to be a very useful histological grading system for accurately predicting lymph node metastasis by IDCs in the no-neoadjuvant therapy group and in the neoadjuvant therapy group.
In a previous study, we found that the grading system for lymph vessel tumor emboli can be used to classify IDC patients with lymph vessel invasion into a low-, intermediate-, and high-risk groups for outcome, and that IDCs with grade 0 lymph vessel tumor embolus and IDCs with grade 1 lymph vessel tumor emboli were almost equally malignant in a different no-neoadjuvant therapy IDC group.6 Although those findings were clearly confirmed in this study again, the results of this study clearly showed that lymph vessel tumor embolus grade 2 in the surgical specimens was an important outcome-predictive factor for IDC patients independent of nodal status. It can be therefore concluded that lymph vessel tumor embolus grade 2 is an important outcome predictor for IDC patients who have received neoadjuvant therapy, the as same as lymph vessel tumor embolus grade 3 is. The results of this study also clearly showed that lymph vessel tumor embolus grades based on biopsy specimens or surgical specimens are a very important outcome-predictive factor for IDC patients who have received neoadjuvant therapy independent of nodal status, but the outcome-predictive power of lymph vessel tumor embolus grade in the surgical specimens was superior to that of lymph vessel tumor embolus grade in the biopsy specimens. Thus, we can conclude that evaluation of lymph vessel tumor embolus grade in surgical specimens should be used to predict outcome.
Although we have already reported that lymph vessel tumor embolus grade is an important outcome predictor for IDC patients who have received neoadjuvant therapy, the outcome-predictive power of the lymph vessel tumor embolus grade for IDC patients who received neoadjuvant therapy and did not have nodal metastasis could not be assessed.7 This study clearly showed that lymph vessel tumor embolus grades based on biopsy specimens and surgical specimens are very important outcome predictors for IDC patients who have received neoadjuvant therapy and do not have nodal metastasis. Furthermore, the outcome-predictive power of lymph vessel tumor embolus grade is almost the same as that of p53 expression in tumor-stromal fibroblasts, and superior to that of histological grade. The lymph vessel tumor embolus grading system is therefore concluded to be an excellent histological grading system for accurately predicting the outcome of IDC patients who have received neoadjuvant therapy that is independent of their nodal status.
The results of this study clearly showed that lymph vessel tumor embolus grades are significantly associated with both the Allred scores for p53 in lymph vessel tumor emboli, as well as the Allred scores for p53 in stroma-invasive tumor cells, and in tumor-stromal fibroblasts, this strongly suggesting that p53 protein expression in lymph vessel tumor emboli, in tumor-stromal fibroblasts, and in stroma-invasive tumor cells is a very important key factor for evaluating the malignant potential of IDCs with lymph vessel tumor emboli. Especially, as lymph vessel tumor embolus grades are based on the numbers of mitotic figures and apoptotic figures in tumor cells in lymph vessels, p53 protein expression in lymph vessel tumor embolus probably accelerates the turnover rate of tumor cells comprising lymph vessel tumor emboli, and increases the malignancy of IDCs as lymph vessel tumor embolus grade rises. As we did not investigate for the presence of p53 gene abnormalities, the mechanism that is responsible for the increase in the malignant potential of IDCs according to grades of lymph vessel tumor embolus from the standpoint of p53 gene abnormalities in lymph vessel tumor emboli, as well as in tumor-stromal fibroblasts, or in stroma-invasive tumor cells should be investigated. In addition, as some studies have reported some identifying genes that closely regulate the cell cycle of tumors,24, 25, 26 such genes should be investigated to determine whether they are candidates for p53 in regulating tumor cell cycle of lymph vessel tumor emboli.
In conclusion, the grading system for lymph vessel tumor emboli is significantly associated with nodal metastasis, and is an excellent histological grading system for accurately predicting the outcome of patients with IDC of the breast who received neoadjuvant therapy. Pathologists can most accurately assess the true malignant potential of IDCs by using this grading system as a histological prognostic classification for IDCs of the breast.
References
Lauria R, Perrone F, Carlomagno C, et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer 1995;76:1772–1778.
Nime FA, Rosen PP, Tzvi Thaler H, et al. Prognostic significance of tumor emboli intramammary lymphatics in patients with mammary carcinoma. Am J Surg Pathol 1977;1:25–30.
Hasebe T, Sasaki S, Imoto S, et al. Characteristics of tumors in lymph vessels play an important role in the tumor progression of invasive ductal carcinoma of the breast: a prospective study. Mod Pathol 2002;15:904–913.
Hoshida T, Isaka N, Hagendoorn J, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelilal growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res 2006;66:8065–8075.
Yamauchi C, Hasebe T, Iwasaki M, et al. Accurate assessment of lymph vessel tumor emboli in invasive ductal carcinoma of the breast according to tumor areas, and their prognostic significance. Hum Pathol 2007;38:247–259.
Hasebe T, Yamauchi C, Iwasaki M, et al. Grading system for lymph vessel tumor emboli for prediction of the outcome of invasive ductal carcinoma of the breast. Hum Pathol 2008;39:427–436.
Tamura N, Hasebe T, Okada N, et al. Tumor histology in lymph vessels and lymph nodes for the accurate prediction of outcome among breast cancer patients treated with neoadjuvant chemotherapy. Cancer Sci 2009;100:1823–1833.
Hasebe T, Tamura N, Okada N, et al. p53 expression in tumor stromal fibroblasts is closely associated with the nodal metastasis and the outcome of patients with invasive ductal carcinoma who received neoadjuvant therapy. Hum Pathol 2009;41:262–270.
Sobin LH, Wittekind Ch . UICC International Union Against Cancer. TNM classification of malignant tumors. In: Sobin LH and Wittekind Ch (eds). 6th edn. Wiley-Liss: Geneva, 2002, pp 131–141.
Bloom HJG, Richardson WW . Histological grading and prognosis in breast cancer. Br J Cancer 1957;11:359–377.
Gilchrist KW, Gray R, Fowble B, et al. Tumor necrosis is a prognostic predictor for early recurrence and death in lymph node-positive breast cancer: a 10-year follow-up study of 728 eastern cooperative oncology group patients. J Clin Oncol 1993;11:1929–1935.
Hasebe T, Tsuda H, Hirohashi S, et al. Fibrotic focus in infiltrating ductal carcinoma of the breast: a significant histopathological prognostic parameter for predicting the long-term survival of the patients. Breast Cancer Res Treat 1998;49:195–208.
Hasebe T, Sasaki S, Imoto S, et al. Prognostic significance of fibrotic focus in invasive ductal carcinoma of the breast: a prospective observational study. Mod Pathol 2002;15:502–516.
Fisher B . Biological and clinical considerations regarding the use of surgery and chemotherapy in the treatment of primary breast cancer. Cancer 1977;40:574–587.
Fisher B . Adjuvant chemotherapy in the primary management of breast cancer. Med Clin North Am 1977;61:953–965.
Fukunaga M . Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 2005;46:396–402.
Niakosari F, Kahn HJ, Marks A, et al. Detection of lymphatic invasion in primary melanoma with monoclonal antibody D2-40: a new selective immunohistochemical marker of lymphatic endothelium. Arch Dermatol 2005;141:440–444.
Allred DC, Harvey JM, Berardo MD, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998;11:155–168.
Harvey JM, Clark GM, Osborne K, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999;17:1474–1481.
Mohsin S, Weiss H, Havighurst T, et al. Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol 2004;17:1545–1554.
Badve SS, Baehner FL, Gray RP, et al. Estrogen- and progesterone-receptor status in ECOG2197: comparison of immunohistochemistry by local and central laboratories and quantitative reverse transcription polymerase chain reaction by central laboratory. J Clin Oncol 2008;26:2473–2481.
Allred DC, Clark GM, Elledge R, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst 1993;85:200–206.
Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18–43.
Westbrook L, Manuvakhova M, Kern FG, et al. Cks1 regulates cdk1 expression: a novel role during mitotic entry in breast cancer cells. Cancer Res 2007;67:11393–11401.
Nakamura Y, Tanaka F, Haraguchi N, et al. Clinicopathological and biological significance of mitotic centromere-associated kinesin overexpression in human gastric cancer. Br J Cancer 2007;97:543–549.
Budhram-Mahadeo VS, Irshad S, Bowen S, et al. Proilferation-associated Brn-3b transcription factor can activate cyclin D1 expression in neuroblastoma and breast cancer cells. Br J Cancer 2008;27:145–154.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (KAKENHI) (C) (19590378, 21590393) from the Japan Society for the Promotion of Science and was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare (20–16, H21-006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hasebe, T., Tamura, N., Iwasaki, M. et al. Grading system for lymph vessel tumor emboli: significant outcome predictor for patients with invasive ductal carcinoma of the breast who received neoadjuvant therapy. Mod Pathol 23, 581–592 (2010). https://doi.org/10.1038/modpathol.2010.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.3
Keywords
This article is cited by
-
Les traitements néoadjuvants TNA (RPC 2013)
Oncologie (2013)
-
Predictive value of pathological and immunohistochemical parameters for axillary lymph node metastasis in breast carcinoma
Diagnostic Pathology (2011)