Abstract
Current pathologic criteria cannot reliably distinguish cutaneous anaplastic large cell lymphoma from other CD30-positive T-cell lymphoproliferative disorders (lymphomatoid papulosis, systemic anaplastic large cell lymphoma with skin involvement, and transformed mycosis fungoides). We previously reported IRF4 (interferon regulatory factor-4) translocations in cutaneous anaplastic large cell lymphomas. Here, we investigated the clinical utility of detecting IRF4 translocations in skin biopsies. We performed fluorescence in situ hybridization (FISH) for IRF4 in 204 biopsies involved by T-cell lymphoproliferative disorders from 182 patients at three institutions. In all, 9 of 45 (20%) cutaneous anaplastic large cell lymphomas and 1 of 32 (3%) cases of lymphomatoid papulosis with informative results demonstrated an IRF4 translocation. Remaining informative cases were negative for a translocation (7 systemic anaplastic large cell lymphomas; 44 cases of mycosis fungoides/Sézary syndrome (13 transformed); 24 peripheral T-cell lymphomas, not otherwise specified; 12 CD4-positive small/medium-sized pleomorphic T-cell lymphomas; 5 extranodal NK/T-cell lymphomas, nasal type; 4 gamma-delta T-cell lymphomas; and 5 other uncommon T-cell lymphoproliferative disorders). Among all cutaneous T-cell lymphoproliferative disorders, FISH for IRF4 had a specificity and positive predictive value for cutaneous anaplastic large cell lymphoma of 99 and 90%, respectively (P=0.00002, Fisher's exact test). Among anaplastic large cell lymphomas, lymphomatoid papulosis, and transformed mycosis fungoides, specificity and positive predictive value were 98 and 90%, respectively (P=0.005). FISH abnormalities other than translocations and IRF4 protein expression were seen in 13 and 65% of cases, respectively, but were nonspecific with regard to T-cell lymphoproliferative disorder subtype. Our findings support the clinical utility of FISH for IRF4 in the differential diagnosis of T-cell lymphoproliferative disorders in skin biopsies, with detection of a translocation favoring cutaneous anaplastic large cell lymphoma. Like all FISH studies, IRF4 testing must be interpreted in the context of morphology, phenotype, and clinical features.
Similar content being viewed by others
Main
Cutaneous CD30-positive T-cell lymphoproliferative disorders include primary cutaneous anaplastic large cell lymphoma (designated heretofore as cutaneous anaplastic large cell lymphoma), lymphomatoid papulosis, transformed mycosis fungoides/Sézary syndrome with CD30 expression, and secondary skin involvement by systemic anaplastic large cell lymphoma. Distinguishing cutaneous anaplastic large cell lymphoma from lymphomatoid papulosis and systemic anaplastic lymphoma kinase- (ALK)-negative anaplastic large cell lymphoma is particularly challenging.1, 2, 3 Histologic and immunophenotypic features of these diseases may be identical, and clinical correlation always is required; in addition, the differential diagnosis with lymphomatoid papulosis sometimes requires lengthy follow-up before a diagnosis can be established.1 Even with follow-up, borderline clinical and pathologic presentations make certain cases difficult to classify. Accurate classification is important, however, because prognosis and therapy differ considerably depending on the diagnosis.4
We recently reported novel translocations involving the IRF4 gene locus in cutaneous anaplastic large cell lymphomas.5 IRF4 encodes interferon regulatory factor-4 (IRF4), also known as multiple myeloma oncogene-1 (MUM1). IRF4 is a transcription factor expressed in activated T cells as well as plasma cells, some B cells, and their corresponding malignant counterparts.6 Using fluorescence in situ hybridization (FISH), we reported the translocation status at the IRF4 locus in 155 peripheral T-cell lymphomas. A majority of translocated cases were cutaneous anaplastic large cell lymphomas (8/14 tested), with occasional translocations detected in peripheral T-cell lymphomas, not otherwise specified (3/64) and systemic ALK-negative anaplastic large cell lymphoma (1/23). Other systemic peripheral T-cell lymphomas, including systemic ALK-positive anaplastic large cell lymphoma, lacked IRF4 translocations. Recently, a French series reported by Pham-Ledard et al7 confirmed the predilection of IRF4 translocations to occur in cutaneous anaplastic large cell lymphomas, and also found translocations in a minority of cases of transformed mycosis fungoides. Taken together, these data suggest the possibility that clinical testing for IRF4 translocations in skin specimens may aid in the classification of T-cell lymphoproliferative disorders. However, neither our previous study5 nor that of Pham-Ledard et al7 was sufficient to determine the clinical role of IRF4 FISH testing because of the limited number of cases, particularly with regard to cutaneous involvement by systemic anaplastic large cell lymphoma and other cutaneous T-cell lymphoproliferative disorders besides cutaneous anaplastic large cell lymphoma, lymphomatoid papulosis, and mycosis fungoides/Sézary syndrome. Therefore, we undertook this multi-institutional study of 204 skin biopsies involved by T-cell lymphoproliferative disorders to determine the specificity of IRF4 translocations for cutaneous anaplastic large cell lymphoma and define the clinical utility of IRF4 FISH in evaluation of cutaneous T-cell lymphoproliferative disorders.
Patients and methods
Patients
We examined 204 skin biopsy specimens from 182 patients diagnosed with cutaneous T-cell lymphoproliferative disorders based on 2008 World Health Organization (WHO) criteria.8 None of the specimens or patients was reported in our previous series.5 All patients were seen and staged by a clinical dermatologist with experience in cutaneous lymphoid disorders. There were 106 men and 76 women (M:F ratio, 1.4:1), with a mean age of 59 years (range, 5–96 years). Diagnoses included: 47 cutaneous anaplastic large cell lymphomas; 44 cases of mycosis fungoides/Sézary syndrome (31 without and 13 with large cell transformation); 32 cases of lymphomatoid papulosis (24 type A, 1 type B, and 7 type C); 25 peripheral T-cell lymphomas, not otherwise specified; 13 CD4-positive small/medium T-cell lymphomas; 5 extranodal NK/T-cell lymphomas, nasal type; 4 gamma-delta T-cell lymphomas; 4 systemic ALK-negative anaplastic large cell lymphomas; 3 systemic ALK-positive anaplastic large cell lymphomas; 3 subcutaneous panniculitis-like T-cell lymphomas; 1 CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma; and 1 T-cell prolymphocytic leukemia. Of the patients in whom multiple biopsies were examined, the same process was seen in all biopsies (one patient had mycosis fungoides in the initial biopsy and transformed mycosis fungoides in the subsequent biopsy). Clinical information included site(s) of disease, status at last follow-up, time to extra-cutaneous spread (in primary cutaneous diseases) and time to death (if reached). The study was approved by the respective Institutional Review Board of each institution providing cases for the study (Mayo Clinic, Cleveland Clinic, and University of Michigan).
Fluorescence In Situ Hybridization
Breakapart FISH for IRF4, localized to 6p25.3, was performed as described previously.5 Briefly, DNA from bacterial artificial chromosome clones (ResGen, Invitrogen, Carlsbad, CA, USA) was isolated using the Plasmid Maxi Kit (Qiagen, Valencia, CA, USA). DNA was labeled either with Texas Red-dUTP (CTD-2308G5, telomeric; Molecular Probes, Invitrogen) or SpectrumGreen-dUTP (RP11-164H16, centromeric; Abbott Molecular, Des Plaines, IL, USA) using the Nick Translation Kit (Abbott). Probe validation and determination of upper limits of normal were described previously.5 Paraffin-embedded whole-tissue sections were digested in 0.4% pepsin solution, hybridized with probe, washed, and counterstained with 4′,6-diamidino 2-phenylindole dihydrochloride. Between 50 and 200 cells per case were analyzed by a microscopist (MEL), with a minimum of 20 abnormal cells required for a sample to be considered abnormal. In some cases, a centromere 6 probe (CEP6 α-satellite SpectrumAqua; Abbott) was applied. In some cases, FISH was combined with CD30 immunofluorescence. Slides were treated with EDTA antigen retrieval, stained with CD30 antibody (Ber-H2, 1:20; Dako, Carpinteria, CA, USA), and detected using biotinylated anti-mouse linker (Dako) and Alexa Fluor 532-labeled streptavidin (Invitrogen). Slides were jet air-dried and hybridized with IRF4 probe as described above.
Immunohistochemistry
IRF4 (MUM1) immunohistochemistry was performed on paraffin-embedded whole-tissue sections as previously reported.5 Briefly, after pretreatment in 1 mM EDTA buffer (pH 8.0) for 30 min at 98 °C (PT Module, LabVision, Fremont, CA, USA), sections were stained using a monoclonal mouse anti-human IRF4 antibody (MUM1p, 1:50; Dako) and signal was detected using Dual-Link Envision+/DAB+ (Dako). Additional immunostains were performed as appropriate to assist in disease classification and IRF4 scoring using antibodies and methods previously published.9, 10 IRF4 was scored as positive (nuclear staining in >30% of tumor cells), partial (10–30%), or negative (<10%). Per 2008 WHO criteria, a diagnosis of cutaneous anaplastic large cell lymphoma required positive CD30 staining in >75% of tumor cells.3
Statistics
Fisher's exact test was used to evaluate differences observed in the frequency of IRF4 translocations between groups. P-values <0.05 were considered statistically significant.
Results
Frequency of IRF4 Translocations in Cutaneous T-cell Lymphoproliferative Disorders
IRF4 FISH was successful in 178 of the 182 patients tested (98%). IRF4 translocations were seen in 10 of the 182 patients (5%), including 9 of 45 (20%) cutaneous anaplastic large cell lymphomas and 1 of 32 (3%) cases of lymphomatoid papulosis (Figure 1a; Table 1). No IRF4 translocation was seen in the other entities tested, including transformed mycosis fungoides (of 13 cases tested: 10 CD30-positive and 3 CD30-negative). One of the nine patients with IRF4-translocated cutaneous anaplastic large cell lymphoma had two biopsies tested, taken 14 months apart: the translocation was present in both biopsies. The percentage of cells with abnormal split FISH signals in translocated cases ranged from 33 to 97% (mean, 62%). These data indicate an overall specificity and positive predictive value of an IRF4 translocation for cutaneous anaplastic large cell lymphoma of 99 and 90%, respectively (P=0.00002; sensitivity, 20%; negative predictive value, 79%). The diseases with morphologic and phenotypic overlap with cutaneous anaplastic large cell lymphoma for which a genetic test would be most helpful include systemic anaplastic large cell lymphoma, lymphomatoid papulosis, and transformed mycosis fungoides.2, 3 Among these entities, the specificity and positive predictive value of an IRF4 translocation for cutaneous anaplastic large cell lymphoma in the current data set are 98 and 90%, respectively (P=0.005; sensitivity, 20%; negative predictive value, 59%). Combining the current data set with data from our previous study5 and from the study of Pham-Ledard et al7 (Table 1), the specificity and positive predictive value for cutaneous anaplastic large cell lymphoma relative to systemic anaplastic large cell lymphoma, lymphomatoid papulosis, and transformed mycosis fungoides are 95 and 88%, respectively (P=0.00007; sensitivity, 28%; negative predictive value, 54%).
Frequencies of IRF4 FISH abnormalities and IRF4 protein expression, stratified by T-cell lymphoproliferative disorder subtype. (a) IRF4 translocations were limited to cutaneous anaplastic large cell lymphomas (20%) and a single case of lymphomatoid papulosis (3%). (b, c) Other IRF4 FISH abnormalities and IRF4 protein expression were widely distributed over T-cell lymphoproliferative disorder subtypes.
Morphologically, differences were not observed between cutaneous anaplastic large cell lymphomas with and without IRF4 translocations. Lesions showed confluent sheets of large tumor cells, often with cytologic features of so-called ‘hallmark’ cells (Figure 2), similar to those seen in systemic anaplastic large cell lymphoma.11 By defining criteria,3 CD30 was expressed in the majority (>75%) of tumor cells in all cases; combined FISH/immunofluorescence in selected cases allowed confirmation that the FISH findings were present in the CD30-positive tumor cells (Figures 3a–c). Similar morphologic and phenotypic characteristics also were seen in the single case of lymphomatoid papulosis with an IRF4 translocation, but the clinical features favored lymphomatoid papulosis (type C) over cutaneous anaplastic large cell lymphoma (see below).
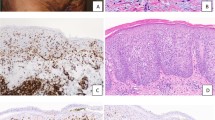
Representative case of primary cutaneous anaplastic large cell lymphoma with IRF4 translocation. (a) A nodular infiltrate expands the dermis (hematoxylin and eosin (H&E), original magnification × 40). (b) At higher power (H&E, × 400), sheets of large tumor cells with abundant cytoplasm are seen. Many have cytologic features of so-called ‘hallmark’ cells (inset; H&E, × 1000). (c, d) By immunohistochemistry ( × 400), the tumor cells are positive for CD30 and IRF4 (MUM1). (e) FISH using an IRF4 breakapart probe shows one normal fusion signal and an abnormal split signal (separated red and green signals), indicating a translocation.
Spectrum of IRF4 FISH abnormalities in cutaneous CD30-positive T-cell lymphoproliferative disorders. Combined FISH/immunofluorescence allows evaluation of the FISH signal pattern in the tumor cells expressing CD30 (gold color): (a) cutaneous anaplastic large cell lymphoma with a normal signal pattern (two fusion signals); (b) cutaneous anaplastic large cell lymphoma with IRF4 translocation (one fusion signal and one split signal); (c) systemic ALK-negative anaplastic large cell lymphoma with extra copy of IRF4 locus (three fusion signals). Addition of CEP6 probe (aqua) allows comparison of copies of IRF4 locus with copies of centromere 6: (d) cutaneous anaplastic large cell lymphoma with IRF4 translocation and two copies of centromere 6; (e) subcutaneous panniculitis-like T-cell lymphoma with seven copies of the IRF4 locus and three copies of centromere 6. Extra proximal (green) signals were seen in three cases and ranged from (f) one extra signal (cutaneous anaplastic large cell lymphoma) to (g) four extra signals (cutaneous anaplastic large cell lymphoma).
Clinical characteristics of the 45 patients with cutaneous anaplastic large cell lymphomas successfully tested for IRF4 translocations are shown in Table 2. Gender and age were similar in patients with and without IRF4 translocations. Translocated cases occurred somewhat more frequently on the head and neck, although this difference was not significant. There was no difference in the incidence of extracutaneous spread (11% of patients) in the two groups; median follow-up was somewhat longer in the untranslocated group (49 months vs 10 months in the translocated group.) Deaths, unrelated to the cutaneous disease in all cases, were similar in both groups. The single lymphomatoid papulosis patient with an IRF4 translocation was a 70-year-old male who presented with a 2-year history of small, spontaneously resolving cutaneous papules. The lesion biopsied was the largest of his skin lesions (1–2 cm in size). Staging studies were otherwise negative, and he remained healthy at last follow-up 4 years after biopsy.
Frequency of Other IRF4 Locus Abnormalities in Cutaneous T-cell Lymphoproliferative Disorders
Other FISH abnormalities were seen in 23 of the 178 cases (13%; Figure 1b). Most of these involved extra copies of the IRF4 locus (21 cases; Figures 3c and e). These cases were mutually exclusive with the translocated cases; that is, cases with translocations did not also demonstrate additional copies of IRF4 (Figure 3d). The number of extra copies ranged from 1 to 5; while some of these extra copies could be attributable to extra copies of chromosome 6, FISH with a centromere 6 probe demonstrated that in some cases the number of intact IRF4 signals exceeded the number of centromeres (Figure 3e). Unlike the presence of IRF4 translocations, the presence of other IRF4 FISH abnormalities was distributed widely over the T-cell lymphoproliferative disorder subtypes tested (Figure 1b). The only patient with multiple biopsies whose FISH results differed between biopsies was a patient with transformed mycosis fungoides in two biopsies taken 19 months apart, with normal FISH in the earlier biopsy and 1–2 extra copies of IRF4 in the later biopsy. In all, IRF4 abnormalities were seen in 3 of 13 cases of transformed mycosis fungoides (23%; all CD30-positive and all involving extra copies of IRF4), but in no case of non-transformed mycosis fungoides/Sézary syndrome (of 31 cases tested). Extra proximal (green) signals were seen in three cases. In two cases (one cutaneous anaplastic large cell lymphoma and one systemic ALK-negative anaplastic large cell lymphoma), there was one extra proximal signal, whereas one case (cutaneous anaplastic large cell lymphoma) had four extra proximal signals (Figures 3f and g). Heterozygous deletion of the IRF4 locus was seen in one case (T-cell prolymphocytic leukemia).
Frequency of IRF4 (MUM1) Protein Expression in Cutaneous T-cell Lymphoproliferative Disorders
A majority of anaplastic large cell lymphomas (85%) were at least partially positive for IRF4 by immunohistochemistry regardless of whether they were cutaneous or systemic and whether or not they expressed ALK (Figure 1c). Among cutaneous anaplastic large cell lymphomas, 70% were positive for IRF4 (including all cases with IRF4 translocations; Figure 2d), and another 14% were partially positive. IRF4 expression also was seen in most cases of lymphomatoid papulosis (71% positive, 25% partial) and transformed mycosis fungoides (75% positive, 8% partial). Varying degrees of IRF4 expression were seen in non-transformed mycosis fungoides/Sézary syndrome; peripheral T-cell lymphoma, not otherwise specified; and other T-cell lymphoproliferative disorders. Among all T-cell lymphoproliferative disorders, IRF4 was positive in 47% of cases and partially positive in another 18%. All patients with multiple biopsies showed concordance of IRF4 expression among their biopsies.
Discussion
In this large, multicenter study, we demonstrate that FISH positivity for an IRF4 translocation is highly specific for cutaneous anaplastic large cell lymphoma in skin biopsies involved by T-cell lymphoproliferative disorders. This finding is important, because current pathologic criteria are insufficient to distinguish cutaneous anaplastic large cell lymphoma from cutaneous involvement by ALK-negative systemic anaplastic large cell lymphoma, lymphomatoid papulosis, and, in some cases, transformed mycosis fungoides.2, 3 The specificity of IRF4 translocations for cutaneous anaplastic large cell lymphoma was 98% among these entities (99% overall). In contrast, IRF4 protein expression was distributed widely across numerous T-cell lymphoproliferative disorder subtypes. These data indicate a clinical role for IRF4 FISH, but not IRF4 immunohistochemistry, in the differential diagnosis of cutaneous T-cell lymphoproliferative disorders.
As discussed above, cutaneous anaplastic large cell lymphoma must be differentiated from cutaneous involvement by systemic ALK-negative anaplastic large cell lymphoma, lymphomatoid papulosis, and transformed mycosis fungoides. Distinguishing between cutaneous anaplastic large cell lymphoma and systemic ALK-negative anaplastic large cell lymphoma currently rests on clinical staging.2, 3 However, even clinical staging is imperfect. For example, the originating tumor site cannot always be determined in patients presenting with both skin lesions and lymphadenopathy. In other cases, only lymph node involvement is apparent to the pathologist and/or clinician; subsequently (or perhaps never), the history of a previous skin biopsy is discovered (which turns out to have been cutaneous anaplastic large cell lymphoma). Correct diagnosis in such cases is important, because the presence of isolated regional lymph node involvement has no significant effect on the prognosis of cutaneous anaplastic large cell lymphoma,4 and the 5-year overall survival of cutaneous anaplastic large cell lymphoma is 90%, compared with 49% in systemic ALK-negative anaplastic large cell lymphoma.12 Several immunohistochemical markers previously have been investigated to distinguish these entities, including clusterin, TRAF1, and IRF4 itself, but none has demonstrated clinical utility in this differential diagnosis.5, 13, 14, 15, 16 Our data suggest that detecting an IRF4 translocation favors a diagnosis of cutaneous anaplastic large cell lymphoma over systemic ALK-negative anaplastic large cell lymphoma in skin biopsies. FISH testing should not, however, replace proper staging. We previously described a single nodal case of systemic ALK-negative anaplastic large cell lymphoma with an IRF4 translocation (of 41 systemic ALK-negative anaplastic large cell lymphomas tested, 2%) (Feldman et al5): although not identified in the current series, such cases theoretically could involve the skin. In addition, we emphasize the importance of ALK testing in cutaneous CD30-positive T-cell lymphoproliferative disorders. Although rare ALK-positive anaplastic large cell lymphomas with clinical features suggesting primary cutaneous disease have been reported,17 the majority of ALK-positive anaplastic large cell lymphomas are systemic.18 In our studies to date, we have not encountered any ALK-positive anaplastic large cell lymphoma with an IRF4 translocation.5
The distinction between cutaneous anaplastic large cell lymphoma and morphologically identical lymphomatoid papulosis is particularly challenging to the pathologist, because it requires an accurate clinical history and/or observation of the patient over time. Lymphomatoid papulosis is characterized by skin lesions that typically wax and wane; however, this history is not always available, depending on the practice setting. Immunohistochemical tests do not aid in this distinction: a single study suggested that IRF4 protein itself could be used to differentiate lymphomatoid papulosis from cutaneous anaplastic large cell lymphoma,19 but there is no evidence for this in this study or in several previous studies.7, 13, 15, 20 Clusterin staining similarly has not been helpful.16, 21 In this study, we detected an IRF4 translocation in a single case of lymphomatoid papulosis, type C (of 31 cases of lymphomatoid papulosis; 3%). This was the only T-cell lymphoproliferative disorder other than cutaneous anaplastic large cell lymphoma to show an IRF4 translocation. This finding may represent genetic evidence for the hypothesis that cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis constitute a clinical and histologic spectrum;1, 22 in fact, classification of ‘borderline’ cases may be difficult, as cutaneous anaplastic large cell lymphoma may regress spontaneously, while type C lymphomatoid papulosis may be histologically indistinguishable from anaplastic large cell lymphoma.3 Our data suggest that, in most cases, the presence of an IRF4 translocation predicts clinical behavior resembling cutaneous anaplastic large cell lymphoma.
By WHO criteria, the diagnosis of cutaneous anaplastic large cell lymphoma requires exclusion of a history of mycosis fungoides.3 Lesions resembling cutaneous anaplastic large cell lymphoma in the context of a history of mycosis fungoides are considered transformed mycosis fungoides. In this study, we did not identify IRF4 translocations in either mycosis fungoides/Sézary syndrome or transformed mycosis fungoides. Pham-Ledard et al reported two cases diagnosed as transformed mycosis fungoides with IRF4 translocations (of 11 tested), both histologically resembling cutaneous anaplastic large cell lymphoma.7 The IRF4 translocation status and clonal relatedness of the original mycosis fungoides lesions were not described. Thus, the possibility that IRF4 translocations represent a transforming event in mycosis fungoides requires further study.
The prognostic significance of IRF4 translocations in cutaneous anaplastic large cell lymphoma remains uncertain. Although we did not find clear differences in outcome between translocated and non-translocated cases, the difference in median follow-up (10 vs 49 months, respectively) and generally short overall follow-up times limit the ability to draw conclusions regarding prognostic significance of the translocation. The possible clinical significance of non-translocation abnormalities by IRF4 FISH (principally extra copies of the intact IRF4 locus) merits study of additional cases with longer follow-up.
Our study confirms earlier findings5, 7 that most cutaneous anaplastic large cell lymphomas express IRF4 protein regardless of the presence of IRF4 translocations. An analogy may be drawn to multiple myeloma, in which IRF4 translocations are present in the minority of cases23 but IRF4 protein is expressed universally.6 Thus, the relationship between IRF4 translocations and IRF4 protein expression is uncertain in both myeloma and cutaneous anaplastic large cell lymphoma. IRF4 has been shown to be critical for myeloma cell growth and represents a potential therapeutic target for that disease.24 Therefore, the role of IRF4 in cutaneous anaplastic large cell lymphoma and related T-cell lymphoproliferative disorders merits further study.
It is possible that the biologic effects of IRF4 translocations are related to neighboring genes on 6p25.3. The IRF4 FISH probe used also flanks the phosphatase gene, DUSP22, located immediately telomeric to IRF4; bacterial artificial chromosomes mapping to the region between IRF4 and DUSP22 cross-hybridize when used for FISH.5, 7 In addition, the gene EXOC2, which encodes a component of the exocyst complex, partially overlaps the centromeric portion of the IRF4 FISH probe. Pham-Ledard et al7 suggested a minor breakpoint region may exist in the IRF4 region involving EXOC2. IRF4 FISH does not distinguish between this possibility and an extra copy of EXOC2 in cases with one extra proximal signal; however, existence of extra copies of EXOC2 is suggested by our case with four extra proximal signals. Finally, biologic significance of IRF4 translocations may derive from the role of the partner gene(s), which remain to be identified.
In summary, we propose that IRF4 FISH is a useful adjunct in the differential diagnosis of cutaneous CD30-positive T-cell lymphoproliferative disorders. In patients without mycosis fungoides/Sézary syndrome, presence of a translocation favors a diagnosis of cutaneous anaplastic large cell lymphoma rather than systemic ALK-negative anaplastic large cell lymphoma or lymphomatoid papulosis. IRF4 FISH represents the most specific test for cutaneous anaplastic large cell lymphoma to date. Like all FISH testing, however, IRF4 FISH should be used in the context of clinical, morphologic, and phenotypic data. The biologic significance of IRF4 translocations and their implication in patients with mycosis fungoides/Sézary syndrome merit further study.
References
Paulli M, Berti E, Rosso R, et al. CD30/Ki-1-positive lymphoproliferative disorders of the skin—clinicopathologic correlation and statistical analysis of 86 cases: a multicentric study from the European Organization for Research and Treatment of Cancer Cutaneous Lymphoma Project Group. J Clin Oncol 1995;13:1343–1354.
Mason DY, Harris NL, Delsol G, et al. Anaplastic large cell lymphoma, ALK-negative. In: Swerdlow S, Campo E, Harris N, et al. (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edn. International Agency for Research on Cancer: Lyon, France, 2008, pp 317–319.
Ralfkiaer E, Willemze R, Paulli M, et al. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow S, Campo E, Harris N, et al. (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4 edn. International Agency for Research on Cancer: Lyon, 2008.
Bekkenk MW, Geelen FA, van Voorst Vader PC,et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood 2000;95:3653–3661.
Feldman AL, Law M, Remstein ED, et al. Recurrent translocations involving the IRF4 oncogene locus in peripheral T-cell lymphomas. Leukemia 2009;23:574–580.
Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 2000;95:2084–2092.
Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-Cell lymphoma: a study of 54 cases. J Invest Dermatol 2010;130:816–825.
Swerdlow S, Campo E, Harris N, et al. (eds) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer: Lyon, France, 2008.
Feldman AL, Law ME, Inwards DJ . et al. PAX5-positive T-cell anaplastic large cell lymphomas associated with extra copies of the PAX5 gene locus. Mod Pathol 2010;23:593–602.
Kurtin PJ, Hobday KS, Ziesmer S, et al. Demonstration of distinct antigenic profiles of small B-cell lymphomas by paraffin section immunohistochemistry. Am J Clin Pathol 1999;112:319–329.
Benharroch D, Meguerian-Bedoyan Z, Lamant L, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 1998;91:2076–2084.
Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496–5504.
Benner MF, Jansen PM, Meijer CJ, et al. Diagnostic and prognostic evaluation of phenotypic markers TRAF1, MUM1, BCL2 and CD15 in cutaneous CD30-positive lymphoproliferative disorders. Br J Dermatol 2009;161:121–127.
Lae ME, Ahmed I, Macon WR . Clusterin is widely expressed in systemic anaplastic large cell lymphoma but fails to differentiate primary from secondary cutaneous anaplastic large cell lymphoma. Am J Clin Pathol 2002;118:773–779.
Wasco MJ, Fullen D, Su L, et al. The expression of MUM1 in cutaneous T-cell lymphoproliferative disorders. Hum Pathol 2008;39:557–563.
Chandra P, Plaza JA, Zuo Z, et al. Clusterin expression correlates with stage and presence of large cells in mycosis fungoides. Am J Clin Pathol 2009;131:511–515.
Kadin ME, Pinkus JL, Pinkus GS, et al. Primary cutaneous ALCL with phosphorylated/activated cytoplasmic ALK and novel phenotype: EMA/MUC1+, cutaneous lymphocyte antigen negative. Am J Surg Pathol 2008;32:1421–1426.
Delsol G, Falini B, Muller-Hermelink HK, et al. Anaplastic large cell lymphoma, ALK-positive. In: Swerdlow S, Campo E, Harris N, et al. (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edn International Agency for Research on Cancer: Lyon, France, 2008, pp 312–316.
Kempf W, Kutzner H, Cozzio A, et al. MUM1 expression in cutaneous CD30+ lymphoproliferative disorders: a valuable tool for the distinction between lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Br J Dermatol 2008;158:1280–1287.
Hernandez-Machin B, de Misa RF, Montenegro T, et al. MUM1 expression does not differentiate primary cutaneous anaplastic large-cell lymphoma and lymphomatoid papulosis. Br J Dermatol 2009;160:713.
Olsen SH, Ma L, Schnitzer B, et al. Clusterin expression in cutaneous CD30-positive lymphoproliferative disorders and their histologic simulants. J Cutan Pathol 2009;36:302–307.
Willemze R, Beljaards RC . Spectrum of primary cutaneous CD30 (Ki-1)-positive lymphoproliferative disorders. A proposal for classification and guidelines for management and treatment. J Am Acad Dermatol 1993;28:973–980.
Iida S, Rao PH, Butler M, et al. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat Genet 1997;17:226–230.
Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature 2008;454:226–231.
Acknowledgements
We would like to acknowledge Dr Joshua Weaver at the Cleveland Clinic and Dr Thomas F Anderson at the University of Michigan for their contributions regarding clinical staging of cases at their respective institutions, and Matthew J Maurer at Mayo Clinic for help with the statistical analysis. ALF is a Damon Runyon Clinical Investigator supported by the Damon Runyon Cancer Research Foundation (CI-48-09). This work was supported in part by a Career Development Award to ALF under Public Health Service Grant number P50 CA097274 from the University of Iowa /Mayo Clinic Lymphoma Specialized Program of Research Excellence (UI/MC Lymphoma SPORE) and the National Cancer Institute. Portions of this study were presented at the 98th Annual Meeting of the United States and Canadian Academy of Pathology, 7–13 March 2009, Boston, MA, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Mayo Clinic and authors MEL, AD, and ALF have a potential financial interest in technology associated with this research. Mayo Clinic has filed a non-provisional patent application for that technology.
Rights and permissions
About this article
Cite this article
Wada, D., Law, M., Hsi, E. et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol 24, 596–605 (2011). https://doi.org/10.1038/modpathol.2010.225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.225
Keywords
This article is cited by
-
Primary cutaneous CD30+ lymphoproliferative disorders with DUSP22 translocation
Die Pathologie (2023)
-
Recent advances in cutaneous lymphoma—implications for current and future classifications
Virchows Archiv (2023)
-
IRF4 drives clonal evolution and lineage choice in a zebrafish model of T-cell lymphoma
Nature Communications (2022)
-
Cutaneous presentation of enteropathy-associated T-cell lymphoma masquerading as a DUSP22-rearranged CD30+ lymphoproliferation
Virchows Archiv (2022)
-
Central nervous system ALK-negative anaplastic large cell lymphoma with IRF4/DUSP22 rearrangement
Brain Tumor Pathology (2022)