Abstract
Well-differentiated liposarcoma/atypical lipomatous tumor can be difficult to differentiate from benign lipomatous tumors, especially on limited biopsy material. Adjunctive tests for MDM2 (murine double minute 2) have proven useful in whole-tissue sections; however, their utility has not been determined within the increasingly popular core needle biopsy. Herein, we compare the ability of MDM2 immunohistochemistry and MDM2 fluorescence in situ hybridization (FISH) to discriminate benign lipomatous tumors from well-differentiated liposarcoma on core needle biopsies. Well-differentiated liposarcoma (n=17) and an assortment of benign lipomatous tumors (n=37), which had concurrent or previous core needle biopsies, and resection specimens were subjected to both MDM2 immunohistochemistry and MDM2 FISH on both whole-tissue sections and corresponding core needle biopsy sections. Percentage tumor cells positive for MDM2 by immunohistochemistry and an MDM2:CEP12 FISH ratio was calculated in each biopsy and resection specimen pair and the results were compared. MDM2 FISH had a higher sensitivity (100%) and specificity (100%) compared with MDM2 immunohistochemistry (65 and 89%) in core needle biopsies, respectively. In addition, MDM2 immunohistochemistry had a false-positive rate of 11%, compared to 0% with FISH. The average MDM2:CEP12 ratio was similar in the biopsy material compared with the whole-tissue sections in both well-differentiated liposarcoma and the benign lipomatous tumor group of neoplasms. Detection of MDM2 amplification by FISH is a more sensitive and specific adjunctive test than MDM2 immunohistochemistry to differentiate well-differentiated liposarcoma from various benign lipomatous tumors, especially on limited tissue samples.
Similar content being viewed by others
Main
Well-differentiated liposarcoma/atypical lipomatous tumor is a locally aggressive mesenchymal neoplasm with varying degrees of mature adipocytic differentiation admixed with atypical stromal cells.1 Well-differentiated liposarcoma is one of the most common sarcomas presenting in adults, usually arising in the retroperitoneum, in the deep soft tissues of the extremities, and in the paratesticular area.2 Lacking metastatic potential, unless it dedifferentiates (dedifferentiated liposarcoma), well-differentiated liposarcoma is potentially curable with complete excision, especially when located in sites such as the deep soft tissue of the extremities wherein a wide margin is feasible. On the other hand, lipomas of deep soft tissue, including the retroperitoneum, though rare, also exist.3 As the biology of these lesions is entirely benign, they can be resected with a minimal margin or some patients might choose not to resect them at all if they are asymptomatic. Therefore, precise recognition by core needle biopsy of lipoma and well-differentiated liposarcoma can facilitate appropriate surgical management, which is important for best patient care practices.
Distinguishing well-differentiated liposarcoma from benign lipomatous tumor mimics such as intramuscular lipoma can be difficult by traditional light microscopy, especially when examining small biopsies (that is, core needle biopsies). The diagnostic atypical, hyperchromatic and enlarged cells found in the lipoma-like variant of well-differentiated liposarcoma may not be sufficiently represented in small biopsies. A brisk inflammatory component characteristic of the inflammatory variant of well-differentiated liposarcoma may also obscure the diagnostic findings.4 Furthermore, morphological findings including pseudolipoblasts, pleomorphic cells, fat necrosis, and intermixed skeletal muscle fibers found in the various benign lipomatous mimickers such as intramuscular lipoma, pleomorphic lipoma, and hibernoma can lead to diagnostic confusion. Thus, the use of adjunctive, relatively objective analytical tools would be useful in these circumstances to help discriminate between these entities, especially in difficult cases or in which there is limited biopsy material such as core needle biopsy.
Well-differentiated liposarcomas have been found by molecular and cytogenetic studies to harbor characteristic ring and giant marker chromosomes containing amplification of the 12q13-15 region, which includes the MDM2 (murine double minute 2) gene.5, 6, 7 MDM2 is an oncogene thought to have a direct role in the pathogenesis of well-differentiated liposarcoma by influencing the cell cycle through degradation of P53.8 Detection of the resultant MDM2 amplification by fluorescence in situ hybridization (FISH) or the corresponding protein overexpression by immunohistochemistry have been shown to be both very sensitive and specific for the distinction of various well-differentiated liposarcoma subtypes from their morphological mimickers.4, 9, 10, 11, 12 However, both adjunctive tools have predominantly been evaluated retrospectively on whole-tissue sections from resection specimens. The utility of both the detection of MDM2 amplification by FISH and MDM2 protein overexpression by immunohistochemistry have not been studied or compared in limited biopsy material/core needle biopsies. Herein, we evaluate their utility and determine whether immunohistochemistry or FISH is better able to detect MDM2 expression and MDM2 amplification, respectively, in a series of core needle biopsies of lipomatous neoplasms. In addition, we designed our study to compare the results of both adjunctive tests in the biopsy/resection specimen pairs from the same patient to make sure that the biopsy results were representative.
Materials and methods
After appropriate local institutional review board approval, various fatty neoplasms recognized in the Department of Anatomic Pathology at the Cleveland Clinic between May 2007 and July 2008 were biopsied at the surgical pathology desk using a 14 g tru-cut trochar with at least three passes or until adequate sample was achieved. Simultaneously, the specimen was processed according to standard soft tissue tumor protocol. Classification of the lipomatous neoplasms was determined by hematoxylin- and eosin-stained tissue sections of the specimens independently reviewed by two soft tissue pathologists (JRG and BPR); discordant cases were reevaluated. Additional cases of well-differentiated liposarcoma with previous core needle biopsies were retrieved from the archives of the Department of Anatomic Pathology at UT-MD Anderson Cancer Center with appropriate local institutional review board approval.
The consensus-verified diagnostic categories included well-differentiated liposarcoma (n=17: 6 from Cleveland Clinic; 11 from UT-MD Anderson), dedifferentiated liposarcoma (n=1), intramuscular lipoma (n=8), superficial lipoma (n=11), fibrolipoma (n=3), angiolipoma (n=10), spindle cell/pleomorphic lipoma (n=2), myelolipoma (n=1), lipoblastoma (n=1), and hibernoma (n=1).
One whole-tissue section and biopsy section from each case were used to perform MDM2 FISH analysis. FISH was performed with a laboratory-developed BAC label probe cocktail from RP11-775J10 and RP11-450G15 BAC DNAs purchased from Roswell Park Cancer Institute, Buffalo, NY, USA specific for MDM2 (12q15) and a probe specific for the centromeric region of chromosome 12 (Abbott Molecular, DesPlaines, IL, USA), as previously described.12 MDM2 FISH was scored blindly as previously described.12 The average number of MDM2 and CEP12 signals was then determined and a MDM2/CEP12 ratio was calculated for each case. A ratio ≥2.0 was considered amplified for the MDM2 gene, whereas a ratio <2.0 was considered nonamplified. A ratio of <2.0 with >2 signals of both probes was considered polysomic for CEP12.
The MDM2 immunohistochemistry was performed on 4-μm thick paraffin-embedded sections on glass Superfrost+ slides using a Discovery XT (Ventana Medical Systems, Tucson, AZ, USA) automated immunohistochemistry instrument with a biotin-free, multimer technology detection kit and conjugate (ChromoMap DAB Kit (760–159)/OmniMap anti-Ms HRP (760–4310), Ventana Medical Systems). For antigen retrieval, CC1 (950–124, Ventana Medical Systems) was used. The antibody was incubated for 1 h at room temperature. The primary MDM2 antibody was from Zymed Laboratories (clone IF2, dilution 1:50). Immunohistochemistry slides were evaluated by two independent soft tissue pathologists (JRG and BPR); discordant cases were reevaluated. MDM2 expression by immunohistochemistry was scored on the basis of percentage of positive lesional nuclei: 0=0%, 1+=1–25%, 2+=26–50%, and 3+=>50%. A tumor was considered MDM2 positive when a score of ≥1+ was assigned. Only nuclear staining was scored as positive.
Results
MDM2 Protein Overexpression by IHC
The MDM2 protein overexpression by immunohistochemistry was shown in most well-differentiated liposarcoma resection sections (n=15/17; 88%), but detected less frequently in the biopsy sections (n=11/17; 65%) (Figure 3). In addition, various benign lipomatous neoplasms (Table 1) had evidence of false-positive MDM2 staining in both biopsy and resection specimens, 11 and 3% of the time, respectively. Further analysis of MDM2 immunohistochemistry revealed that most of the positive cases (57% of well-differentiated liposarcomas and 100% of benign lipomatous tumors) exhibited only 1+ immunoreactivity (Table 2).
MDM2 Amplification by FISH
MDM2 amplification by FISH was present in 11/11 biopsy and 16/17 resection specimens (Figure 4). The average MDM2:CEP12 ratio within the well-differentiated liposarcoma group was 4.6. None of the various benign lipomatous neoplasms studied (Table 1) showed amplification of MDM2 by FISH. The average MDM2:CEP12 ratio within the benign lipomatous neoplasm group was 0.9. Autofluorescence was present in 11 of 54 (20%) biopsy sections, compared with only 2 of 54 (4%) resection tissue sections.
Comparison of Adjunctive Tests and Biopsy Versus Resection Tissue Sections
The sensitivity, specificity, positive predictive value, and negative predictive value were all higher for the detection of MDM2 amplification by FISH compared with the detection of MDM2 protein expression by immunohistochemistry (Table 3). The test indices of MDM2 immunohistochemistry were all significantly lower in biopsies, as compared with resection specimens, whereas they were similar when MDM2 FISH was used (Table 2). The average MDM2:CEP12 ratios within the well-differentiated liposarcoma group in biopsy and resection specimens were 4.7 and 4.6, respectively. The average MDM2:CEP12 ratios within the benign lipomatous neoplasm group in the biopsy and resection specimens were both 0.9.
Discussion
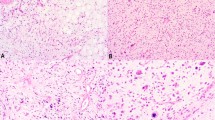
Well-differentiated liposarcoma can be difficult to distinguish from benign lipomatous lesions, especially on limited material (that is, core needle biopsies) in which the diagnostic features of scattered atypical cells are not present because of the heterogeneity of these neoplasms (Figures 1 and 2). Detection of MDM2 protein expression by immunohistochemistry or MDM2 amplification by FISH as surrogates for the marker chromosomes characteristic of well-differentiated liposarcoma have been shown to be helpful adjunctive tools when performed on whole-tissue sections of well-characterized cases.4, 9, 10, 11, 12 However, distinguishing a benign lipomatous lesion from well-differentiated liposarcoma is most important at primary biopsy, more commonly being performed by computer topography-guided core needle biopsies, in which limited diagnostic material is obtained. It is in these instances wherein it is most important to understand the limitations of the adjunctive tests described above. Herein, we have shown that both the detection of MDM2 protein overexpression by immunohistochemistry, although less sensitive and specific, and the detection of MDM2 gene amplification by FISH can be used on formalin-fixed, paraffin-embedded core needle biopsies of difficult fatty tumors (Figures 1, 2, 3 and 4).
Whole-tissue section of the same well-differentiated liposarcoma depicted in Figure 1 showing diagnostic thick fibrous bands with pleomorphic spindled cells (hematoxylin and eosin staining, × 10).
MDM2 immunostaining performed on the core needle biopsy from the liposarcoma depicted in Figure 1 showing rare tumor cell positivity (1+) ( × 10).
Performing MDM2 immunohistochemistry to detect protein overexpression and MDM2 FISH to detect gene amplification on concurrent biopsies and whole-tissue sections of well-differentiated liposarcoma and benign lipomatous lesions allowed comparison of these adjunctive tests. The MDM2 FISH assay is a more sensitive and specific test for well-differentiated liposarcoma, especially on limited biopsy material. The superiority of MDM2 FISH on biopsy samples results from the fact that MDM2 amplification is present in both morphologically atypical and nonatypical lesional cells. In addition, the accuracy of MDM2 FISH on biopsy samples is remarkable when the average MDM2:CEP2 ratio is compared with the ratio obtained on whole-tissue sections. However, autofluorescence due to fixation remains to be a technical issue with the MDM2 FISH assay, especially in the small core needle biopsies. In total, 20% of the biopsies in this study were not able to be evaluated owing to autofluorescence. This is a well known limitation of FISH.13 As this technology improves, it is likely that the problem of autofluorescence will be reduced or solved altogether.
The MDM2 immunohistochemistry performance drops when dealing with limited tissue material. This phenomenon can be attributed to the focal (1+, <25% tumor cells) staining within the majority of well-differentiated liposarcomas (Table 2). These foci of MDM2 positive tumor cells may not be present in small core needle biopsies because of limited sampling. The sensitivity improves when evaluating whole-tissue sections, further supporting the above theory. In addition, the specificity of the MDM2 immunostaining assay suffers because 11% of the benign lipomatous tumors in this study had some degree of MDM2 protein expression on the biopsy material, whereas no MDM2 amplification by FISH was noted in any of the benign lipomatous tumors. FISH seems to be more robust and accurate than MDM2 immunohistochemistry, especially in core needle biopsy specimens.
In conclusion, well-differentiated liposarcoma can mimic benign lipomatous tumors because of the absence of characteristic morphological features that are not sampled on small biopsy material. Detection of MDM2 amplification by FISH is a more sensitive and specific adjunctive test than MDM2 immunohistochemistry to differentiate these entities, especially on limited tissue samples.
References
Dei Tos AP, Pedeutour F . Atypical lipomatous tumour/well differentiated liposarcoma. In: Fletcher CD, Unni, KK, Mertens, F, (eds). World Health Organization Classification of Tumours Pathology & Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, 2002, pp 35–37.
Weiss SW, Goldblum JR . Liposarcoma. Enzinger and Weiss's Soft Tissue Tumors. St Louis: Mosby, 2008, pp 477–516.
Macarenco RS, Erickson-Johnson M, Wang X, et al. Retroperitoneal lipomatous tumors without cytologic atypia: are they lipomas?: a clinicopathologic and molecular study of 19 cases. Am J Surg Pathol 2009;33:1470–1476.
Weaver J, Goldblum JR, Turner S, et al. Detection of MDM2 gene amplification or protein expression distinguishes sclerosing mesenteritis and retroperitoneal fibrosis from inflammatory well-differentiated liposarcoma. Mod Pathol 2009;22:66–70.
Dal Cin P, Kools P, Sciot R, et al. Cytogenetic and fluorescence in situ hybridization investigation of ring chromosomes characterizing a specific pathologic subgroup of adipose tissue tumors. Cancer Genet Cytogenet 1993;68:85–90.
Karakousis CP, Dal Cin P, Turc-Carel C, et al. Chromosomal changes in soft-tissue sarcomas. A new diagnostic parameter. Arch Surg 1987;122:1257–1260.
Pedeutour F, Suijkerbuijk RF, Forus A, et al. Complex composition and co-amplification of SAS and MDM2 in ring and giant rod marker chromosomes in well-differentiated liposarcoma. Genes Chromosomes Cancer 1994;10:85–94.
Vargas DA, Takahashi S, Ronai Z . Mdm2: A regulator of cell growth and death. Adv Cancer Res 2003;89:1–34.
Binh MB, Garau XS, Guillou L, et al. Reproducibility of MDM2 and CDK4 staining in soft tissue tumors. Am J Clin Pathol 2006;125:693–697.
Binh MB, Sastre-Garau X, Guillou L, et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol 2005;29:1340–1347.
Sirvent N, Coindre JM, Maire G, et al. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol 2007;31:1476–1489.
Weaver J, Downs-Kelly E, Goldblum JR, et al. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol 2008;21:943–949.
Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Weaver, J., Rao, P., Goldblum, J. et al. Can MDM2 analytical tests performed on core needle biopsy be relied upon to diagnose well-differentiated liposarcoma?. Mod Pathol 23, 1301–1306 (2010). https://doi.org/10.1038/modpathol.2010.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.106
Keywords
This article is cited by
-
A scoring system combining clinical, radiological, and histopathological examinations for differential diagnosis between lipoma and atypical lipomatous tumor/well-differentiated liposarcoma
Scientific Reports (2022)
-
Nuclear expression of MDM2 in hibernoma: a potential diagnostic pitfall
Virchows Archiv (2021)
-
Accuracy of histological grades from intraoperative frozen-section diagnoses of soft-tissue tumors
International Journal of Clinical Oncology (2020)
-
Pilot study to differentiate lipoma from atypical lipomatous tumour/well-differentiated liposarcoma using MR radiomics-based texture analysis
Skeletal Radiology (2020)
-
Well-Differentiated Liposarcoma (Atypical Lipomatous Tumor) Presenting as an Esophageal Polyp
Journal of Gastrointestinal Cancer (2019)