Abstract
The diagnosis of gastric epithelial dysplasia, a precursor lesion of gastric adenocarcinoma, is hindered by interobserver variability and by its resemblance to regenerative changes. Loss of cell polarity, a histological feature of gastric epithelial dysplasia, may be difficult to ascertain, especially in the setting of inflammation or injury. A biomarker of cell polarity could be useful in diagnosis of dysplasia, but has not been reported. The Lethal giant larvae (lgl) gene controls apical–basal polarity of epithelial cells in Drosophila, and has properties of a tumor-suppressor gene. Two homologs, lgl1 and lgl2, are present in mammals and lgl2 mRNA is highly expressed in the stomach. The goal of our study was to test the hypothesis that Lgl2 protein expression and/or localization are disrupted in gastric epithelial dysplasia and adenocarcinoma. Routinely processed pathology specimens including 94 benign mucosae of digestive organs, in addition to 36 reactive gastropathy, 57 gastric epithelial dysplasia, and 77 gastric adenocarcinomas, were immunostained for Lgl2 protein. All normal, reactive, and chronically inflamed gastric epithelia showed basolateral Lgl2 staining. Normal esophageal, duodenal, colonic, biliary, and pancreatic duct mucosae, as well as gastric intestinal metaplasia, did not express Lgl2. All but one case each of gastric epithelial dysplasia and adenocarcinoma showed either complete loss of anti-Lgl2 immunoreactivity or diffuse, mostly weak, cytoplasmic staining. Complete loss of immunoreactivity was significantly more often observed in diffuse-type than in intestinal-type adenocarcinomas (79 vs 48%, respectively). Our data suggest that Lgl2 expression is either aberrantly localized or lost in gastric epithelial dysplasia and adenocarcinoma, whereas it is maintained in reactive gastric mucosa. We propose that Lgl2 may be a potential marker to rule out gastric epithelial dysplasia and adenocarcinoma in diagnostic specimens. However, the consistently negative anti-Lgl2 immunoreactivity seen in intestinal metaplasia does not allow differentiation of dysplasia from intestinal metaplasia with reactive change.
Similar content being viewed by others
Main
The histological diagnosis of gastric epithelial dysplasia, a precursor lesion of gastric adenocarcinoma, can be challenging. It is hampered by several factors, including interobserver variability, and, at the low end of the spectrum, its resemblance to reactive changes.1 Among the histological criteria, loss of cell polarity of the epithelium is one of the more objective markers of dysplasia. It is usually defined as stratification of nuclei in apical–basal direction (low-grade dysplasia) or loss of the perpendicular orientation of the long axes of the nuclei in relation to the basal membrane (high-grade dysplasia). Other, less precise, terms used to describe disrupted cell polarity include disordered sheets of epithelial cells and cell crowding. However, these features may be mild and difficult to ascertain morphologically in low-grade dysplasia, and more so in the setting of inflammation or injury, because reparative changes can show similar alterations. Evidently, utilization of a biomarker to demonstrate disrupted cell polarity would be a helpful adjunct in the diagnosis of gastric epithelial dysplasia.
Lethal giant larvae (lgl), first discovered in Drosophila, is a gene involved in the maintenance of epithelial cell polarity and asymmetric cell division.2 lgl encodes a protein that associates with the submembranous actin cytoskeleton of the basolateral cell domain.3, 4, 5 Due to the unique ability of its homozygous mutation in Drosophila to simultaneously disrupt cell polarity and induce formation of malignant tumors, lgl was defined as a tumor-suppressor gene.2, 5, 6 Two homologs, lgl1 (also known as hugl-1) and lgl2, are present in humans, but little is known about the function of lgl in human malignancies. Because it has been demonstrated that lgl2 mRNA is highly expressed in mammalian stomach,7 we sought to evaluate whether Lgl2 protein expression or localization is disrupted in gastric dysplasia and carcinoma. Accordingly, we studied the pattern of Lgl2 immunostaining in normal epithelia of human digestive organs in reactive gastropathy, gastric epithelial dysplasia, and gastric adenocarcinoma. Our results show that Lgl2 expression is either lost or exhibits aberrant localization in almost all cases of gastric epithelial dysplasia and adenocarcinomas.
Materials and methods
Case Selection
Paraffin blocks with normal, reactive, and neoplastic mucosa of digestive organs in resection, endoscopic mucosal resection, or biopsy specimens were obtained from the surgical pathology files of UMass Memorial Medical Center (Worcester, MA, USA) and Massachusetts General Hospital (Boston, MA, USA). They included 94 specimens of normal mucosa (esophagus: 8; stomach: 41 (antrum: 14; corpus/fundus: 14; and cardia: 13), duodenum: 11; colon: 12; common bile duct: 15; pancreatic duct: 7); biopsies of chemical gastropathy: 36; gastric epithelial dysplasia: 21 polypoid dysplasia (ie, adenomas; 16 biopsies, 5 endoscopic mucosal resections), 36 flat dysplasia (11 endoscopic mucosal resection, 25 adjacent to intestinal-type gastric cancer in resection specimens); 77 gastric adenocarcinomas: 44 intestinal type, including 5 biopsies, 24 diffuse type, and 9 other types. All specimens had been formalin fixed, paraffin embedded, and processed routinely. Approval for the study was obtained from the institutional review boards.
Histological and Immunohistochemical Analysis
Reactive gastropathy was diagnosed using currently accepted criteria.8, 9 Gastric epithelial dysplasia was classified as low grade (crowded tubular glands lined by atypical columnar cells with overlapping, penicillate, hyperchromatic nuclei, pseudostratification, and inconspicuous nucleoli) or high grade (crowded, branching, and budding glands lined by cuboidal cells with high nuclear to cytoplasmic ratio, round to oval vesicular nuclei with prominent nucleoli, and distinct loss of nuclear polarity).1, 10, 11 Lauren's classification was used to categorize gastric adenocarcinomas as intestinal or diffuse type.12 Intestinal-type adenocarcinomas were subclassified into well differentiated (well-formed glands or papillae), moderately differentiated (irregularly branching glands or complex and incomplete papillae), and poorly differentiated (ill-formed glands or single infiltrative cells13).
To evaluate the expression of Lgl2 protein, we optimized the staining protocol using several different tissues. Normal and tumor samples from liver, kidney, colon, testis, and stomach were first used to explore the distribution of the protein throughout organ systems. Two different anti-Lgl2 antibodies, mouse polyclonal and monoclonal antibody from Novus Biologicals (Littleton, CO, USA), and a variety of concentrations and staining conditions were used on each of these to determine the nature of the protein expression. As predicted from animal model studies, intracellular basolateral distribution of the Lgl2 protein in the stomach epithelium was demonstrated. The selected monoclonal antibody (see below) was titrated out to achieve the best resolution of basolateral staining from the background. Titration was performed using normal and hyperplastic antral mucosa with the cutoff being where basolateral staining began to be lost. For each case, 1–4 blocks were selected for immunostaining. Immunostaining was performed on 5-μm sections. Antigen retrieval was carried out with 0.01 M citrate buffer (pH 6.0) in a 770-W microwave oven for 14 min. The slides were stained on the Dako Autostainer (Dako Corporation, Carpinteria, CA, USA) using the EnVision+ (Dako Corporation) staining reagents. The sections were first blocked for endogenous nonspecific protein and peroxidase activity with an application of Dual Endogenous Block (Dako Corporation) for 10 min, followed by a buffer wash. They were then incubated with anti-Lgl2 antibody at 1:750 dilution (mouse monoclonal, clone 4G2; Novus Biologicals) for 30 min, followed by a buffer wash. After incubation with the EnVision+ Dual Link (Dako Corporation) detection reagent (a polymer conjugated with goat anti-mouse Ig, goat anti-rabbit IgG, and horseradish peroxidase) for 30 min, the sections were washed and treated with a solution of diaminobenzidine and hydrogen peroxide for 10 min. After rinsing, a toning solution (DAB Enhancer; Dako Corporation) was used for 5 min to enrich the final coloration. Counterstaining was achieved with hematoxylin. Negative controls were stained using the same procedure, but with antibody diluent only in place of the primary antibody. Patterns of Lgl2 expression were scored as basolateral, cytoplasmic, and negative (no immunoreactivity present). When more than one pattern was observed in a specimen, major and minor patterns of staining were recorded. The immunoreactivity pattern was qualified as major if it was present in 50% or more of the cells of interest; a minor pattern of staining was defined as present in less than 50% of the cells of interest. The intensity of staining was semiquantitatively scored as weak, moderate, or strong.
Statistical Analysis
Differences in the proportion of nonreactive specimens between diffuse- and intestinal-type gastric adenocarcinomas were evaluated by Fisher's exact test. The association between tumor grade and staining pattern was evaluated using the Freeman–Halton extension of the Fisher's exact test to R × C tables.14 Differences in overall survival were evaluated using Kaplan–Meier Product-Limit survival analysis.15 Differences in overall survival between grades and between patterns were evaluated using the Tarone–Ware test.15
Results
Clinicopathological Features
On the basis of Lauren classification, we observed that 44 gastric adenocarcinomas were of the intestinal type, 24 were of diffuse type, and 9 of various patterns (mixed type: 5; mucinous type: 2; and with lymphoid stroma/medullary: 2). Six adenocarcinomas originated in the cardia, and the remainder were from the antral/pyloric (n=51) and body/fundic regions (n=20). Of 21 gastric adenomas, 6 had areas of invasive carcinoma. The patients’ demographic features are shown in Table 1.
Patterns of Lgl2 Expression in Normal Gastrointestinal and Pancreatobiliary Duct Mucosa
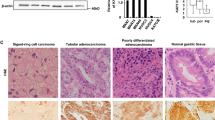
All specimens of normal gastric epithelia, including 14 antrum, 14 corpus, and 13 cardia, showed basolateral anti-Lgl2 immunoreactivity. The expression was strong in cells at the luminal surface and the upper part of the gastric pits, and it was moderate/weak toward the base of the pits and in the glands (Figure 1a and b). Notably, the apical cell surface and the cytoplasm did not stain. Epithelium of the antral/cardiac and oxyntic glands showed a similar pattern of expression, although often weaker and more difficult to evaluate due to gland coiling (Figure 1c and d). In contrast, 8 normal esophageal, 11 duodenal, 12 colonic, 15 common bile duct, and 7 pancreatic duct mucosa showed no immunoreactivity for Lgl2 (Figure 1c and d). Lamina propria, submucosa, and muscularis propria were uniformly negative. Overall, these findings demonstrate that Lgl2 immunoreactivity selectively marks gastric epithelium in the digestive tract.
(a–d) Anti-Lgl2 monoclonal antibody reacts with gastric epithelium in basolateral fashion. (a) Antral mucosa, (b) foveolar epithelium, (c) antral glands, and (d) oxyntic glands. No reactivity is seen in the duodenum (e) or the colon (f). Basolateral pattern of staining is preserved in reactive gastropathy (g and h).
Patterns of Lgl2 Expression in Reactive Gastropathy, Chronic Gastritis, and Intestinal Metaplasia
All of the 36 cases of reactive gastropathy showed a basolateral pattern of staining for Lgl2 with absence of staining at the apical cell surface and in the cytoplasm, similar to normal mucosa (Figure 1e and f).
Areas of chronic inactive gastritis were present in all 77 cases of gastric adenocarcinomas and invariably showed a basolateral pattern of Lgl2 immunoreactivity. These results suggest that a normal pattern of Lgl2 staining is preserved in reactive gastropathy and in chronic inactive gastritis.
Areas of intestinal metaplasia were present in 51 gastric adenocarcinomas. Similar to the epithelium of the small and large bowel, they did not show Lgl2 expression.
Patterns of Lgl2 Expression in Low-Grade Gastric Dysplasia
Lgl2 immunoreactivity was significantly decreased or lost in all but one case of gastric adenoma. Of the 21 cases of gastric adenomas (19 intestinal type and 2 foveolar/gastric type), 20 (95%) showed absence of staining as the only or major pattern (Figure 2a and b; Table 2). Both foveolar/gastric-type adenomas stained negatively for Lgl2. In one case (n=1; 5%), the immunostaining was basolateral.
Loss of anti-Lgl2 immunoreactivity in gastric dysplasia and adenocarcinoma. (a and b) Low-grade dysplasia; (c and d) high-grade dysplasia; (e and f) gastric adenocarcinoma of intestinal type (tumor, left side of the panels; center, intestinal metaplasia; foveolar hyperplasia, right side of the panels; insets show intestinal metaplasia and foveolar epithelium at higher magnification); (g and h) gastric adenocarcinoma of diffuse type.
Lgl2 staining was either lost or exhibited aberrant localization in all cases of flat low-grade gastric epithelial dysplasia. Twelve cases, including 2 examples of foveolar/gastric-type dysplasia and 10 foci adjacent to intestinal-type gastric adenocarcinoma, were available for analysis. Lgl2 staining was negative in 8 (67%) specimens, including both cases of foveolar/gastric-type dysplasia. Weak cytoplasmic staining was the only or major pattern in four (33%) cases (Table 2).
Patterns of Lgl2 Expression in High-Grade Gastric Dysplasia
All specimens with high-grade dysplasia showed loss or aberrant localization of Lgl2 immunoreactivity. Of 23 cases available for analysis, 17 foci were adjacent to gastric adenocarcinoma and 3 were part of adenoma. Twenty had an adenomatous phenotype and three a foveolar/gastric phenotype. Absence of anti-Lgl2 staining was the only or major pattern in 16 (69%) cases, including 1 case of foveolar/gastric-type dysplasia (Figure 2c and d; Table 2). Cytoplasmic staining of variable (weak to strong) intensity was present as the only or major pattern in seven (31%) specimens, including two cases of foveolar/gastric type (Table 2).
Patterns of Lgl2 Expression in Gastric Adenocarcinoma
Anti-Lgl2 immunoreactivity was lost or was present in an aberrant location in the majority of 44 gastric adenocarcinomas of intestinal type. In this group, negative staining was observed in 30 (69%) cases as the only or major pattern (Figure 2e and f; Table 2). Cytoplasmic staining of variable (weak to strong) intensity was the only or major pattern in 13 (29%) cases. Basolateral anti-Lgl2 staining in combination with minor cytoplasmic pattern was seen in one (2%) well-differentiated gastric adenocarcinoma (Table 2). Of four other well-differentiated gastric adenocarcinomas, three were negative and one had cytoplasmic staining. The staining pattern of the invasive front of carcinomas did not differ significantly from the more superficial part of the tumors.
Lgl2 was not expressed or showed abnormal localization in all 24 gastric adenocarcinomas of diffuse type. Negative anti-Lgl2 immunoreactivity was the only or major pattern in 23 (96%) cases (Figure 2g and h; Table 2). Weak diffuse staining was a major pattern in one (4%) case.
Absence of anti-Lgl2 staining was the only or major pattern in six cases (67%) of gastric adenocarcinomas of other morphological types. Weak diffuse staining was the only or major pattern in three (33%) other cases (Table 2).
Statistical Analysis
The proportion of specimens that are nonreactive for Lgl2 in the diffuse-type gastric adenocarcinoma group was significantly higher than in the intestinal-type adenocarcinoma group (79 and 48%, respectively; P=0.0194; Table 2). No significant association was found between the grade or stage of intestinal-type adenocarcinoma and anti-Lgl2 staining pattern. Follow-up data were available for 26 patients with intestinal-type adenocarcinoma. There were no significant differences in survival function found between staining patterns in patients with intestinal-type gastric adenocarcinoma (P=0.693). Follow-up data were available for 20 patients with diffuse-type gastric adenocarcinoma, and 18 of them had negative staining. Therefore, analysis of survival function was not performed.
Discussion
The stable cytoarchitecture of normal differentiated epithelia depends on the preserved apical–basal polarity of constituent cells. Alteration or loss of cell polarity is a structural change that commonly occurs during malignant transformation. Recent characterization of the molecular basis of cell polarity allows its function in neoplasia to be deciphered. It has now been demonstrated that interdependent activity of three protein complexes determines apical and basolateral membrane domains in epithelial cells. The most apical is the Crumbs–Stardust–Patj complex.16 Another complex, including atypical protein kinase C (aPKC), Par3, and Par6, localizes to tight junctions and overlaps with the apical Crumbs-containing region.16 Lgl, Scribble (Scrib), and Disc large (Dlg) are part of the basolateral domain and act in the same genetic pathway.5 Lgl is a downstream target of aPKC that, once phosphorylated, dissociates from the actin cytoskeleton and thereby is excluded from the apical domain.17, 18 Mutual inhibition of aPKC and Lgl has been shown to maintain complementary apical and basal cortical domains.19, 20 Mutations of lgl, scrib, or dlg in Drosophila lead not only to loss of polarized cell phenotype, but also to hyperproliferation, tumor formation, invasiveness, broad dispersal of tumor cells reminiscent of metastasis and tumor transplantability, all characteristics of a malignant neoplasm.2, 6 That loss of cell polarity is a significant factor in malignant progression was also shown in a genetic screen performed in Drosophila to find mutations sufficient to transform noninvasive tumors into invasive ones. Expression of activated RasV12 in clones of cells of the eye disk produced noninvasive tumors. However, coupled inactivation of any of a number of cell polarity genes led to invasion and secondary tumor formation.21, 22
In mice, targeted disruption of the lgl1 gene resulted in abnormal cell polarity in embryonic brain neuroepithelium associated with increased proliferation, lack of differentiation, and formation of rosette-like structures reminiscent of human primitive neuroectodermal tumor, suggesting that the cell polarity function of lgl is evolutionarily conserved.7 Furthermore, human lgl1 transgene was used to reverse malignant mutant lgl phenotype in Drosophila, indicating that human lgl1 has the properties of a tumor-suppressor gene.23 Indirectly supporting this view, lgl1 mRNA was reduced or absent in 62% of 60 human solid tumors composed of breast, lung, prostate, and ovarian carcinomas and melanomas;23 in 60% of 10 colonic adenomas; and in 75% of 94 colorectal carcinomas.24 No data on the function of lgl2 in human malignancies have been reported so far.
In this study, we showed that the cell polarity protein Lgl2 was expressed in normal gastric epithelium but was absent in small and large intestine, biliary and pancreatic ducts, and esophagus. Lgl2 expression was limited to the basolateral cell domain, consistent with localization of Lgl in epithelial cells of model organisms.19, 25 The intensity of staining progressively increased from the base of the gastric pits, where less-differentiated cells are located, to the mucosal surface, suggesting that Lgl2 expression is a feature of more differentiated cells.
To evaluate whether Lgl2 expression can be used to distinguish gastric epithelial dysplasia or adenocarcinoma from regenerative/reactive change, we first examined a series of cases with reactive gastropathy and areas of chronic gastritis in specimens harboring gastric epithelial dysplasia or adenocarcinoma. All gastropathy specimens and all areas of chronic gastritis showed basolateral staining similar to normal epithelium. In contrast, the overwhelming majority of gastric dysplasia and adenocarcinoma showed abnormalities of Lgl2 immunoreactivity ranging from diffuse cytoplasmic misplacement to complete loss of staining. More than one immunostaining pattern could be present in a neoplasm, suggesting inter- and intratumoral variability of Lgl2 expression. One explanation for different staining patterns could be the variable degrees of neoplastic differentiation. Indeed, negative staining was observed significantly more often in diffuse gastric adenocarcinomas than in the intestinal-type adenocarcinomas. However, there was no correlation between the grade of intestinal-type adenocarcinoma and staining pattern.
Our study attests to early abnormality of Lgl2 expression in gastric neoplastic progression and supports the notion that lgl2 might function as a human tumor-suppressor gene. The molecular basis of abnormal Lgl2 expression and localization in gastric adenocarcinoma is not known. Activation of the intestinal differentiation program is a plausible explanation for the absence of Lgl2 immunostaining in intestinal-type gastric epithelial dysplasia and adenocarcinoma (a pattern similar to small and large bowel as well as intestinal metaplasia). However, intestinal differentiation cannot explain the loss or aberrant localization in foveolar (gastric)-type dysplasia and in diffuse-type adenocarcinoma, neither of which is known to have close association with intestinal differentiation. Moreover, intestinal differentiation per se does not lead to cytoplasmic localization of anti-Lgl2 immunoreactivity, a frequent finding in intestinal-type dysplasia and adenocarcinoma. It is likely that dysregulation of signaling pathways controlling Lgl2 expression and/or inactivating mutations of the lgl2 gene is involved in these cases.
Constitutive activation of the Wnt signaling pathway has been implicated in gastric carcinogenesis.26, 27, 28 It might be significant that ectopic activation of Wnt signaling interferes with membranous localization of Lgl, with concomitant loss of its activity.25 This effect is mediated by Disheveled, an essential intracellular mediator of Wnt signaling, which associates with Lgl and is required for its stability and proper localization.25
In summary, the localization of Lgl2 to the basolateral cell domain in normal gastric epithelium is consistent with its proposed function in control of cell polarity. We have shown that the loss of expression of Lgl2 or its aberrant localization is closely associated with gastric epithelial dysplasia and gastric adenocarcinoma. One caveat on using this potential biomarker is the consistently negative anti-Lgl2 immunoreactivity observed in intestinal metaplasia. Consequently, absence of Lgl2 staining cannot distinguish dysplasia from intestinal metaplasia with reactive change. We conclude that Lgl2 could be a useful marker diagnostically to rule out gastric epithelial dysplasia or adenocarcinoma. Additional studies are warranted to advance our understanding of the function of Lgl2 in pathogenesis of gastric epithelial dysplasia and gastric adenocarcinoma.
References
Lauwers GY, Srivastava A . Gastric preneoplastic lesions and epithelial dysplasia. Gastroenterol Clin North Am 2007;36:813–829.
Gateff E . Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 1978;200:1448–1459.
Albertson R, Doe CQ . Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol 2003;5:166–170.
Betschinger J, Knoblich JA . Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol 2004;14:R674–R685.
Bilder D . Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev 2004;18:1909–1925.
Bilder D, Li M, Perrimon N . Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 2000;289:113–116.
Klezovitch O, Fernandez TE, Tapscott SJ, et al. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev 2004;18:559–571.
Sobala GM, King RF, Axon AT, et al. Reflux gastritis in the intact stomach. J Clin Pathol 1990;43:303–306.
Quinn CM, Bjarnason I, Price AB . Gastritis in patients on non-steroidal anti-inflammatory drugs. Histopathology 1993;23:341–348.
Lauwers GY, Riddell RH . Gastric epithelial dysplasia. Gut 1999;45:784–790.
Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol 2000;24:167–176.
Lauren P . The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31–49.
Fenoglio-Preser C, Carneiro F, Correa P, et al. Tumors of the stomach. In: Hamilton SR, Aaltonen LA (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. IARC Press: Lyon, France, 2000, pp 37–52.
Freeman GH, Halton JH . Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 1951;38:141–149.
Hosmer DW, Lemeshow S . Applied Survival Analysis. John Wiley & Sons Inc.: New York, NY, 1999.
Assémat E, Bazellières E, Pallesi-Pocachard E, et al. Polarity complex proteins. Biochim Biophys Acta 2008;1778:614–630.
Plant PJ, Fawcett JP, Lin DC, et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol 2003;5:301–308.
Yamanaka T, Horikoshi Y, Sugiyama Y, et al. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol 2003;13:734–743.
Chalmers AD, Pambos M, Mason J, et al. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development 2005;132:977–986.
Hutterer A, Betschinger J, Petronczki M, et al. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell 2004;6:845–854.
Brumby AM, Richardson HE . Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J 2003;22:5769–5779.
Pagliarini RA, Xu T . A genetic screen in Drosophila for metastatic behavior. Science 2003;302:1227–1231.
Grifoni D, Garoia F, Schimanski CC, et al. The human protein Hugl-1 substitutes for Drosophila lethal giant larvae tumour suppressor function in vivo. Oncogene 2004;23:8688–8694.
Schimanski CC, Schmitz G, Kashyap A, et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene 2005;24:3100–3109.
Dollar GL, Weber U, Mlodzik M, et al. Regulation of lethal giant larvae by Dishevelled. Nature 2005;437:1376–1380.
Clements WM, Wang J, Sarnaik A, et al. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 2002;62:3503–3506.
Horii A, Nakatsuru S, Miyoshi Y, et al. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res 1992;52:3231–3233.
Nojima M, Suzuki H, Toyota M, et al. Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene 2007;26:4699–4713.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lisovsky, M., Dresser, K., Baker, S. et al. Cell polarity protein Lgl2 is lost or aberrantly localized in gastric dysplasia and adenocarcinoma: an immunohistochemical study. Mod Pathol 22, 977–984 (2009). https://doi.org/10.1038/modpathol.2009.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.68
Keywords
This article is cited by
-
Rewiring cell polarity signaling in cancer
Oncogene (2015)
-
Deregulation of the cell polarity protein Lethal giant larvae 2 (Lgl2) correlates with gastric cancer progression
Gastric Cancer (2014)
-
The human Lgl polarity gene, Hugl-2, induces MET and suppresses Snail tumorigenesis
Oncogene (2013)
-
Epithelial cell polarity and tumorigenesis: new perspectives for cancer detection and treatment
Acta Pharmacologica Sinica (2011)
-
Loss of cell polarity protein Lgl2 in foveolar-type gastric dysplasia: correlation with expression of the apical marker aPKC-zeta
Virchows Archiv (2010)