Abstract
Urothelial papillomas and low-grade urothelial carcinomas have shown a high incidence of fibroblast growth factor receptor 3 (FGFR3) mutations and are associated with a favorable prognosis. The association of FGFR3 mutations with inverted papillomas is less known. We analyzed 20 cases of inverted papilloma in the urinary tract. Mutations of FGFR3 (exons 7, 10, and 15) and TP53 genes were evaluated by DNA sequencing in these cases. Point mutations of the FGFR3 gene were identified in 45% (9 of 20) of inverted papillomas with four cases exhibiting mutations at multiple exons. Seven cases had exon 7 mutations containing R248C, S249T, L259L, P260P, and V266M. Two cases had exon 10 and 15 mutations including A366D, H412H, E627D, D641N, and H643D; five cases had N653H. The most frequent mutation was identified at R248C. None of the inverted papillomas exhibited mutations in TP53. During a mean follow-up of 78 months, none had recurrence or developed urothelial carcinoma. These findings support the concept that low-grade and low-stage urothelial neoplasms arise in a background of molecular changes that are distinctly different from the molecular changes of high-grade and high-stage urothelial cancers.
Similar content being viewed by others
Main
The fibroblast growth factor receptor 3 (FGFR3) is a member of a family of tyrosine kinase receptors and is composed of an extracellular ligand-binding domain, a transmembrane region, and a cytoplasmic domain with tyrosine kinase activity. Ligand binding causes receptor dimerization and subsequent activation of intracellular tyrosines. Activating mutations of FGFR3 gene lead to constitutive activation of the receptor subsequently inducing the downstream molecular pathogenesis. Activating point mutations of FGFR3 have been associated with autosomal dominant dwarfism and severe achondroplasia. An oncogenic role for FGFR3 in human cancer has emerged recently and FGFR3 mutations were reported to be associated with multiple myeloma, urothelial, and cervical cancers.
Urothelial carcinomas harboring FGRFR3 mutations, in general, tend to be of low histological grade and of low pathological stage, and consequently are associated with a more favorable clinical outcome.1, 2, 3 Inverted papillomas of the urinary tract are uncommon. If strict histological criteria are adhered to in making the diagnosis, their biological behavior is almost invariably benign.4 Although a number of studies have addressed FGFR3 mutation status in urothelial carcinoma, there is a paucity of information concerning FGFR3 abnormalities in inverted papilloma of the urinary tract. Because inverted papillomas lack aggressive biological behavior, and FGFR3 mutation appears to be associated with relatively indolent behavior in low-grade and low-stage urothelial carcinomas, we tested the frequency and specific types of FGFR3 mutations, in conjunction with TP53 mutation status, in 20 cases of inverted papilloma.
Materials and methods
Tumors Samples and Microdissection
Twenty urothelial lesions diagnosed as inverted papilloma were analyzed. All the cases were reviewed retrospectively and fulfilled the diagnostic criteria using the 2004 WHO classification of the genitourinary tumors.5 None had prior or current urothelial carcinoma in situ (CIS) or papillary transitional cell carcinoma (urothelial carcinoma).
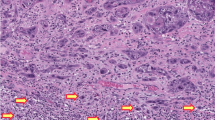
Paraffin-embedded tissue was collected from each of the 20 cases of inverted papilloma. Histological sections were prepared from formalin-fixed, paraffin-embedded tissue. The slides were stained with hematoxylin and eosin (HE) for microscopic evaluation after deparaffinization with xylene and ethyl alcohol. Laser-assisted microdissection of the tumor tissues was performed on the lightly HE-stained sections using a PixCell II Laser Capture Microdissection System (Arcturus Engineering, Mountain View, CA, USA).6, 7 Approximately 600–1000 cells of each tumor were microdissected from the 4-μm histological sections as demonstrated in Figure 1. Microdissected normal tissue from the same patient served as a control. The dissected tissue was incubated in 50 μl of digesting buffer containing 10 mM Tris-HCl, 1 mM EDTA, 1% Tween 20, and 5 mg/ml of proteinase K (pH 8.3) at 37°C overnight. The samples were boiled for 10 min to inactivate proteinase K. The genomic DNA from each sample was dissolved in 30 μl of dd H2O after phenol–chloroform extraction (phenol/chloroform/isoamyl alcohol=25:24:1).
Urothelial inverted papilloma (case 4). (a) Laser microdissection of inverted papilloma. (a1) Lesion (inverted papilloma) before microdissection. (a2) Lesion after microdissection. (a3) Laser capture lesional cells. (b) FGFR3 gene mutation detected by direct sequencing. Upper panel: normal tissue (control); lower panel: point mutation at exon 10, A366D, GCT → GAT. (c) FGFR3 gene mutation detected by direct sequencing. Upper panel: normal tissue (control); lower panel: point mutation at exon 15, N653H, AAC → CAC.
FGFR3 Mutation Analysis
Exons 7, 10, and 15 of FGFR3 were amplified by PCR using the previously reported primers.8, 9, 10 PCR was performed with 3 μl of isolated genomic DNA in a final volume of 50 μl containing 2.3 mM MgCl2, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 μM deoxynucleotide triphosphates, 2 μM each primer, and 2 U Taq DNA polymerase (Bio-Rad, Hercules, CA, USA). Each PCR protocol had an initial denaturing step of 95°C for 5 min, followed by 40 cycles at 95°C for 30 s, at 55°C (exons 7 and 15) for 30 s or at 58°C (exon 10) for 30 s, and at 72°C for 30 s, and then followed by a single final extension step at 72°C for 7 min. The PCR products were purified by QIAquick PCR Purification kit (Qiagen Sciences, Germantown, MD, USA). DNA concentration of PCR products was measured and adjusted to 20 ng per microliter. Purified PCR product was sequenced by ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
TP53 Mutation Analysis
TP53 DNA in exons 5, 7, and 8 was amplified by PCR using the established primers.11, 12, 13 PCR was performed with 3 μl of isolated genomic DNA in a final volume of 50 μl containing 2.3 mM MgCl2, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 μM deoxynucleotide triphosphates, 2 μM each primer, and 2 U Taq DNA polymerase (Bio-Rad). Each PCR protocol had an initial denaturing step of 95°C for 5 min, followed by 40 cycles at 95°C for 30 s, at 55°C for 30 s, and at 72°C for 30 s, and then followed by a single final extension step at 72°C for 7 min. The PCR products were purified by QIAquick PCR Purification kit (Qiagen Sciences). DNA concentration of PCR products was measured and adjusted to 20 ng per microliter. Purified PCR product was sequenced by ABI Prism 3100 Genetic Analyzer.
Results
The inverted papillomas were diagnosed according to accepted criteria.5 The median age at diagnosis was 58 years (range 37–75 years). Eighteen of the patients were men whereas two were women. The most common location of the lesion was the bladder neck (5 of 20 cases), whereas lateral wall (3 of 20), trigone (4 of 20), ureteral orifice (2 of 20), ureter (4 of 20), and base and dome (1 of 20 each) were also reportedly involved. The most common presenting complaint was hematuria. The mean clinical follow-up was 78 months (range 11–220 months). None developed recurrence or urothelial carcinoma.
FGFR3 mutation analysis was performed in all tumors. The mutations detected in inverted papillomas included R248C, S249T, L259L, P260P, and V266M at exon 7; A366D and H412H at exon10; E627D, D641N, H643D, and N653H at exon 15 (Table 1; Figures 1 and 2). Previously described missense mutations and activating mutations that were detected include R248C and S249T.14 To the best of our knowledge, mutations of L259L, P260P, V266M, A366D, H412H, E627D, D641N, H643D, and N653H were not reported in previously published papers. Missense or candidates of activating mutations that were detected include V266M, A366D, E627D, D641N, H643D, and N653H. Finally, the L259L, P260P, and H412H mutations are silent mutations, probably of no significant biological impact. Overall, 9 of 20 inverted papillomas (45%) demonstrated point mutations, with 2 identified in three cases and 3 mutations in one case. The most common mutation was R248C, which was observed in three cases. D641N mutations were identified in two cases. The other mutations were found only once in separate cases.
Schematic illustration of FGFR3 mutation positions in the inverted urothelial papillomas. Mutations were presented as the type of mutation and amino-acid changes designated to the FGFR3 protein structure. Ig I, Ig II, and Ig III: immunoglobulin-like domains; TM: transmembrane domain; TK-1 and TK-2: tyrosine kinase domains. Red-coded mutations are activating mutations published previously; blue-coded are missense mutations that are candidate of activating mutations; green-coded are silent mutations.
No mutations were identified in normal tissue from the same specimen in patients with inverted papilloma. No mutations in TP53 genes were identified in any inverted papillomas. Although more studies are needed to determine whether the mutations identified in this series have a causative effect on the development of inverted papillomas, the presence of the mutations within the papillomas and the absence in normal tissue support this hypothesis.
Discussion
The FGFR3 gene on chromosome 4p16 is involved in cell signaling pathways and angiogenesis as well as cell proliferation, development, and differentiation. FGFR3 mutations are associated with autosomal dominant skeletal disorders such as achondroplasia and thanatophoric dysplasia.15 More recently, FGFR3 mutations have been identified in some human neoplasms, including multiple myeloma and carcinoma of the uterine cervix, but not in neoplasms of the stomach, rectum, colon, prostate, ovary, breast, brain, lung, skin, esophagus, or kidney.16 In this report, we found a high frequency of FGFR3 mutations in inverted urothelial papilloma.
A strong association between FGFR3 mutation and low-grade urothelial carcinoma has been recognized.1, 17 Indeed, FGFR3 mutations are associated with low-stage, low-grade tumors. FGFR3 mutations have also been identified in 23% of flat hyperplastic urothelial lesions (7 of 30).18 A recent study of inverted papillomas of the urinary tract revealed infrequent FGFR3 mutations (9.8% of cases).19 Specific codons involved in point mutations in urothelial carcinomas include 248, 249, 372, 375, and 652.15, 20 Tumors harboring more than one FGFR3 mutation have been identified.10, 21 FGFR3b-S249C, the most common mutation in bladder tumors, was found to be tumorigenic in vitro and also gave rise to tumors in mice that were xenografted with FGFR3b-S249C transfected cells.22
Two divergent molecular pathways appear to exist in urothelial tumorigenesis and cancer progression (Table 2). Higher-grade tumors are much less likely to harbor FGFR3 mutations; in contrast, they often exhibit TP53 mutations.20 Low-grade papillary tumors typically harbor activating mutations of FGFR3. These tumors tend to be genetically stable even if they do frequently recur. They are often multifocal and may arise from simple urothelial hyperplasia, and usually do not progress to invasion. An alternate molecular pathway is believed to be operative in the carcinogenesis of a group of urothelial carcinomas with entirely different clinical and pathological features, characterized by aggressive behavior and a tendency to be invasive. These tumors are frequently associated with TP53 mutations, which appear early in tumorigenesis. They typically arise de novo and are frequently found in high-grade, flat, and CIS tumors, usually with no previous history of low-grade, noninvasive papillary lesions. These tumors are genetically unstable and tend to accumulate mutations. The TP53 mutations lead to dysfunction in cell cycle and apoptosis. Mutations in TP53, but not FGFR3, are known to be influenced by smoking. FGFR3 and TP53 mutations have not been identified in normal bladder epithelium from patients with confirmed urothelial carcinoma, suggesting the mutations were somatic.15, 23 Additional molecular studies by Jebar et al,9 have found that FGFR3 mutations are mutually exclusive of Ras gene mutations in urothelial cell carcinoma (Table 2).
Although the concept of two divergent pathways to tumorigenesis is appealing, consideration should be given to the possibility of genetic progression leading to increased aggressiveness in urothelial carcinomas. Specifically, FGFR3 mutations may signal an early event in tumorigenesis followed by TP53 mutation. However, analyses of both high- and low-grade tumors have only yielded a relatively small number of tumors harboring both TP53 and FGFR3 mutations.1, 13 van Rhijn et al1 hypothesized that an intermediate phenotype in urothelial carcinomas with both FGFR3 and TP53 alterations may exist.
In our study, 45% of inverted papillomas exhibited mutations in the FGFR3 gene, whereas none harbored TP53 mutations. These findings support the concept that low-grade and low-stage urothelial neoplasms arise in a background of molecular changes that are distinctly different from the molecular changes preceding the onset of high-grade, invasive, biologically aggressive bladder cancers.
The exact mechanism by which activating mutations lead to tumorigenesis is still being deciphered. The FGFR3 regulates cell growth and differentiation through the banding of fibroblast growth factors. The mechanism of FGFR3-mutation-related oncogenesis is believed to cause constitutive activation of the receptor. van Rhijn et al1 identified activating point mutations of FGFR3 in low-grade urothelial carcinomas by sequencing. Exons 7, 10, and 15 seem especially susceptible to tumor-initiating mutations.13, 14 FGFR3 mutations were found only in papilloma but not in adjacent normal tissue, suggesting a causative affect.
In addition to identifying two previously described mutations (S249T and R248C), we found several new missense and candidate activating mutations (Figure 2). These mutations include V266M, E627D, A366D, D641N, H643D, and N653H. We also identified three silent mutations (L259L, P260P, and H412H) in inverted papillomas. In conclusion, FGFR3 mutations are associated with inverted urothelial papillomas. Benign and low-grade papillary urothelial lesions including ‘hyperplasia’, papillomas, inverted papillomas, PUNLMP, and low-grade papillary urothelial carcinomas may well result from a similar etiology. TP53 gene mutations were not associated with inverted papillomas.
References
van Rhijn BW, van der Kwast TH, Vis AN, et al. FGFR3 and P53 characterize alternative genetic pathways in the pathogenesis of urothelial cell carcinoma. Cancer Res 2004;64:1911–1914.
Hernandez S, Lopez-Knowles E, Lloreta J, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol 2006;24:3664–3671.
Hernandez S, Lopez-Knowles E, Lloreta J, et al. FGFR3 and Tp53 mutations in T1G3 transitional bladder carcinomas: independent distribution and lack of association with prognosis. Clin Cancer Res 2005;11:5444–5450.
Sung MT, Maclennan GT, Lopez-Beltran A, et al. Natural history of urothelial inverted papilloma. Cancer 2006;107:2622–2627.
Eble JN, Sauter G, Epstein JI, et al. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press: Lyon, 2004.
Cheng L, Zhang S, Wang M, et al. Molecular genetic evidence supporting the neoplastic nature of stromal cells in ‘fibrosis’ after chemotherapy for testicular germ cell tumors. J Pathol 2007;213:65–71.
Cheng L, MacLennan GT, Zhang S, et al. Laser capture microdissection analysis reveals frequent allelic losses in papillary urothelial neoplasm of low malignant potential of the urinary bladder. Cancer 2004;101:183–188.
Kimura T, Suzuki H, Ohashi T, et al. The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer 2001;92:2555–2561.
Jebar AH, Hurst CD, Tomlinson DC, et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 2005;24:5218–5225.
Tomlinson DC, Baldo O, Harnden P, et al. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol 2007;213:91–98.
van der Sijp JR, van Meerbeeck JP, Maat AP, et al. Determination of the molecular relationship between multiple tumors within one patient is of clinical importance. J Clin Oncol 2002;20:1105–1114.
Vet JA, Bringuier PP, Schaafsma HE, et al. Comparison of P53 protein overexpression with P53 mutation in bladder cancer: clinical and biologic aspects. Lab Invest 1995;73:837–843.
Bakkar AA, Wallerand H, Radvanyi F, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res 2003;63:8108–8112.
Wallerand H, Bakkar AA, de Medina SG, et al. Mutations in TP53, but not FGFR3, in urothelial cell carcinoma of the bladder are influenced by smoking: contribution of exogenous versus endogenous carcinogens. Carcinogenesis 2005;26:177–184.
Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol 2001;158:1955–1959.
Sibley K, Stern P, Knowles MA . Frequency of fibroblast growth factor receptor 3 mutations in sporadic tumours. Oncogene 2001;20:4416–4418.
van Oers JM, Lurkin I, van Exsel AJ, et al. A simple and fast method for the simultaneous detection of nine fibroblast growth factor receptor 3 mutations in bladder cancer and voided urine. Clin Cancer Res 2005;11:7743–7748.
van Oers JM, Adam C, Denzinger S, et al. Chromosome 9 deletions are more frequent than FGFR3 mutations in flat urothelial hyperplasias of the bladder. Int J Cancer 2006;119:1212–1215.
Eiber M, van Oers JM, Zwarthoff EC, et al. Low frequency of molecular changes and tumor recurrence in inverted papillomas of the urinary tract. Am J Surg Pathol 2007;31:938–946.
van Rhijn BW, Montironi R, Zwarthoff EC, et al. Frequent FGFR3 mutations in urothelial papilloma. J Pathol 2002;198:245–251.
van Rhijn BW, Lurkin I, Chopin DK, et al. Combined microsatellite and FGFR3 mutation analysis enables a highly sensitive detection of urothelial cell carcinoma in voided urine. Clin Cancer Res 2003;9:257–263.
Bernard-Pierrot I, Brams A, Dunois-Larde C, et al. Oncogenic properties of the mutated forms of fibroblast growth factor receptor 3b. Carcinogenesis 2006;27:740–747.
Lindgren D, Liedberg F, Andersson A, et al. Molecular characterization of early-stage bladder carcinomas by expression profiles, FGFR3 mutation status, and loss of 9q. Oncogene 2006;25:2685–2696.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lott, S., Wang, M., Zhang, S. et al. FGFR3 and TP53 mutation analysis in inverted urothelial papilloma: incidence and etiological considerations. Mod Pathol 22, 627–632 (2009). https://doi.org/10.1038/modpathol.2009.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.28