Abstract
Tumor stage and grade for gastrointestinal stromal tumors are poorly defined. To develop a better evaluation system, we assessed 12 clinical and pathological parameters in 613 patients with follow-up information. These parameters were classified into two gross spread parameters including liver metastasis and peritoneal dissemination, five microscopic spread parameters including lymph node metastasis, vascular, fat, nerve and mucosal infiltration, and five histological parameters including mitotic count ≥10 per 50 high-power fields, muscularis propria infiltration, coagulative necrosis, perivascular pattern and severe nuclear atypia. The 5-year disease-free survival and overall survival of 293 patients without any of these predictive parameters of malignancy were 99 and 100%, respectively. They were regarded as nonmalignant and further evaluations on the stage and grade of these tumors were not performed. At least one and at most seven predictive parameters of malignancy were identified in 320 patients. For these patients, the 5-year disease-free survival and overall survival rates were 44% (mean 6.7 years) and 60% (mean 9.3 years), respectively. The disease-free survival showed significant difference between patients with and without gross spread (P<0.0001), with and without microscopic spread (P=0.0009). Disease-free survival and overall survival were associated with the number of predictive parameters of malignancy in patients without gross spread (P<0.0001 for both disease-free survival and overall survival), but not in patients with gross spread (P=0.882 and 0.441, respectively). Malignant gastrointestinal stromal tumors could be divided into clinical stage I and II based on the absence and presence of gross spread, respectively. The degree of malignancy of patients in clinical stage I could be graded according to the number of predictive parameters of malignancy. Patients in clinical stage II were of the highest degree of malignancy regardless of the number of parameters. We found that the clinical stage and grade were strongly associated with prognosis.
Similar content being viewed by others
Main
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract. Recent progress in the understanding of the molecular mechanisms of their oncogenesis has contributed to the improvements in their diagnosis and treatment.1, 2 The discovery that almost all GISTs express KIT/CD117 antigen has led to the development of imatinib mesylate (imatinib, Glivec; Novartis, Basel, Switzerland) for the targeted therapy of GISTs.3 Several clinical trials have shown the effectiveness of imatinib in the treatment of advanced, metastatic and recurrent GISTs. The life expectancy of GIST patients has dramatically increased after treatment with imatinib. Although most patients with unresectable or metastatic GIST benefit from imatinib treatment, clinical resistance of this drug is a significant problem.4 The median time to disease progression is about 18 or 20 months when treated at 400 or 800 mg dose levels.5, 6 Therefore, the best treatment regimen for patients with malignant GISTs, like doing imatinib preoperatively or postoperatively, is still under debate.7, 8, 9
The guideline for the selection of patients for adjuvant therapy varies among experts, mainly due to the criteria predicting patients with a high risk of recurrence after the surgical removal of primary GISTs have yet to be established. Clinically, some patients with malignant GIST are highly aggressive, developing recurrence within short time after surgical removal of the primary tumor, whereas others can be treated effectively by surgical resection alone or had a long latency to develop recurrence. Many investigators made efforts to grade10, 11, 12, 13, 14, 15 and/or stage GISTs.11, 16, 17, 18 On the basis of these previous reports and our preliminary study, we selected 12 parameters that not only had predictive value for malignancy, but also had value to stage and grade GISTs effectively.19 We introduced a new and simple method based on these parameters for the staging and grading of GISTs. The objective of this study was to systematically correlate the clinical outcome of a large number of GIST patients with 12 predictive parameters of malignancy and obtain an effective grading and staging system for GISTs. Our new approach was also compared with the NIH consensus criteria and it could be valuable for the design of treatments for GIST patients.
Materials and methods
Tumor Specimens
Medical records and tissue specimens of 1155 primary mesenchymal tumors of GI tract were retrieved from 12 hospitals in Shanghai. The hospitals and the years patients were treated were Zhongshan Hospital, Fudan University, 1993–2006; Cancer Hospital, 1985–2002; Huashan Hospital, 1980–1999; Huadong Hospital, 1981–2000; Changning Centre Hospital, 1992–2002; Zhabei Centre Hospital, 1996–2003; Yangpu Center Hospital, 1990–2004; Chongming Centre Hospital, 1980–2001; Qinpu Centre Hospital, 1971–2000; Putuo Centre Hospital, 1983–2000; The Tenth People's Hospital, 1998–2003 and Gongli Hospital, 1996–2003. The 1155 cases were primary mesenchymal tumors previously characterized as leiomyoma, leiomyosarcoma, leiomyoblastoma, schwannoma, stromal or smooth muscle tumors originated from GI tract, 771 of 1155 cases that underwent surgery were immunohistochemically or histologically identified as GISTs based on the positive immunohistochemical detection of KIT or the identical histopathological spectrum with KIT-positive tissues. All tumor slides were reviewed by two experienced pathologists. Another 69 GIST patients were collected from our own consultant file from January 2005 to March 2007. The institutional review boards approved tumor tissue collection and the following analyses.
Clinical Records
Patient demographics and clinical data were retrieved from the medical records. Parameters selected for analyses were the following: age (classified as <50 years or ≥50 years), sex, complaints and main symptoms, tumor size (stratified as <5, ≥5 to <10, and ≥10 cm), tumor site (stomach, duodenum, small intestine, rectum and others including esophagus, colon, extra-GI tract and unspecified), predominant growth pattern (chiefly submucosa, predominantly intramural, mainly outgrowth and others including extra-GI as well as unspecified), presence of ulceration, adhesion, tumor rupture, pedicle, liver metastases, peritoneal dissemination and surgical procedures (with curative or palliative intention).
Histological Evaluation
A total of 1-21 hematoxylin and eosin (H&E)-stained slides (with a median of 4 slides) for each patient were reviewed and the following features were recorded: predominant cell type, pleomorphism, nuclear atypia, necrosis, perivascular pattern, mitotic count and invasion.
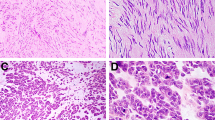
Predominant cell type in a tumor tissue refers to the cell type constituting greater than 70% of the total cells, including spindle cells, epithelioid cells or mixed type of cells.20 The severity of pleomorphism of the tumor cells was defined by nuclear atypia and classified by the previously described criteria21 with modifications. No or mild pleomorphism indicates that the sizes of tumor cell nuclei show no or little difference from those of normal cells, or the average size of nuclei is roughly equal to one lymphocyte in diameter; moderate pleomorphism indicates some enlargement of the size of the nuclei equaling approximately to two lymphocytes in diameter; severe pleomorphism indicates obvious changes of nuclear sizes, or significant number of atypical nuclei are enlarged to a size transversely equal to three lymphocytes in diameter. The severity of nuclear atypia can be further classified as focal or diffused.22, 23 A few cases of scattered focal and severe atypia were combined into the group of moderate atypia for statistical analysis, and diffused and severe nuclear atypia were classified as the severe group (Figure 1). Necrosis was classified as coagulate necrosis, when ghost of tumor cells was identified in the necrotic area21 (Figure 2). Perivascular pattern was identified when tumor cells packed and grew around vessels as perivascular collars23, 24 (Figure 3). Mitotic count is the number of mitotically active cells in 50 consecutive high-power fields at a magnification of × 400 using an Olympus BX41 microscope with × 40 objective and × 10 ocular lens (0.159 mm2). All slides were first examined for the most proliferate areas before counting the mitotic cells. All mitotic cells counted in 50 high-power fields were recorded as <5/50 high-power fields, or 5–10/50 high-power fields and ≥10/50 high-power fields.
The tumor-invaded tissues were identified under a light microscope by the previously reported methods11, 20, 21, 25, 26 with modifications. Muscular propria infiltration or ‘muscle infiltration’ indicates the presence of tumor cells between smooth muscle fibers as tongue-like, nest-like or sheet structures, and the fibers were splayed or dissected by tumor cells27, 28 (Figure 4). Mucosal infiltration was registered when tumor cells infiltrated inside the normal epithelial layers23, 27, 28 (Figure 5). Invasion of fat29, 30 (Figure 6) or nerve tissues (Figure 7), vascular infiltration or tumor emboli31 (Figure 8) and lymph node metastasis were all recorded. Pathological changes in the tumor stromal structures (cystic, hemorrhagic) were also recorded. Slides were reviewed by experienced pathologists who were blind to the patients' medical records or their disease outcomes.
Immunochemical Evaluation
Immunohistochemical staining was performed based on previously reported method.23 Formalin-fixed paraffin sections were prepared from one representative block and subjected to immunohistochemical staining with a panel of antibodies against CD117 (rabbit polyclonal anti-human c-KIT, diluted 1:150; Dako, Denmark), CD34 (mouse monoclonal antibody, clone QBEnd 10, diluted 1:200; Dako), α-smooth muscle actin (mouse monoclonal antibody, clone 1A4, diluted 1:200; Dako), desmin (mouse monoclonal antibody, clone D33, diluted 1:200; Dako) and S-100 protein (polyclonal, diluted 1:300; Dako). The slides were first treated with 0.01 M citrate buffer (pH 6.0) by microwave method for antigen retrieval, and incubated overnight at 4°C. Immunohistochemical detection was performed with EnVision- and avidin-biotin-based polymer system using a commercial kit (Dako). Diaminobenzidine was used as the chromogen, and all sides were counterstained with hematoxylin.
Patient Follow-up Information
The follow-up information for patients after surgeries and treatments was provided by the referring pathologists and clinicians, or obtained directly from patients and their family members.
Classification and Statistic Analysis
Among 840 patients, 181 patients with curative resection and 5 patients with palliative resection were lost during the follow-up because of relocation or telephone number change, 24 patients without recurrence or death with less than 1-year follow-up were not included in survival analysis, and 33 patients with palliative resection and 7 patients died of other reasons within 1-year were not included in disease-free survival analysis. Therefore, 250 patients were excluded from the disease-free survival analysis. In the end, 590 patients were evaluated for disease-free survival. Disease-free survival and overall survival were measured from the time of surgery to the time of first recurrence or most recent follow-up or death.
For the overall survival analysis, similar patient selection criteria were used, except patients with palliative resection were included and patients with imatinib therapy after recurrence were excluded. In the end, 562 patients were evaluated for overall survival. The total number of patients that underwent disease-free survival and overall survival was 613.
Statistic analysis was carried out using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). Kaplan–Meier method was used to calculate the disease-free survival and overall survival functions. Log-rank test and Cox regression were used for univariate and multivariate analyses. P<0.05 was considered statistically significant.
Results
Clinical and Pathological Features
Of the patients, 373 were male and 240 were female. The patient age range was from 17 to 84 (median, 58 years). The patients most commonly presented with GI bleeding (206 patients), which was most often insidious bleeding with anemia and weakness. The other symptoms were abdominal pain or uncomfortable abdomen, and 86 patients had abdominal mass. In 127 patients, the tumor was incidentally detected during other medical procedure, for example, for physical examination (n=57) and other abdominal surgery (n=70). The median tumor size was 5 cm (range, 0.2–35), and 271 patients were with tumor <5 cm, 187 with tumor ≥5 to <10 cm and 155 with tumor ≥10 cm. There were 335 gastric tumors, 37 duodenal, 141 small intestinal, 60 rectal and 40 at another primary site (10 esophagus, 7 colon, 12 extra-GI tract, and 2 multiple sites and 9 unspecified). 421 were predominantly intramural, 19 were chiefly submucosal, 173 were mainly outgrowth or out of GI tract. 178 patients had adhesion and 25 patients had tumor rupture at operation. Thirteen patients had liver metastases, 22 had peritoneal dissemination and 4 had both liver metastases and peritoneal dissemination.
A majority of the GISTs were spindle-cell tumors (504, 82%). Epithelioid morphology was seen in 47 cases (8%). There were mixed spindle cell and epithelioid component in 62 patients (10%). Tumors exhibited mild to moderate cellularity or mild to moderate nuclear atypia. The degree of cellularity and atypia often varied at different areas of the same tumor. There was no or mild atypia in 136 cases, moderate atypia in 348 cases and severe atypia in 129 cases. Mitotic rates varied from 0 to >100 per 50 high-power fields (mean, 17 mitoses per 50 high-power field). There were <5 per 50 high-power fields in 364 patients (59%), ≥5 to <10 per 50 high-power fields in 54 (9%) patients, ≥10 per 50 high-power field in 195 patients (32%).
Ulceration was common and present in 175 of 613 cases (29%). Coagulative necrosis was present in 150 (25%) cases, and muscle infiltration was present in 197 cases (32%). A perivascular growth pattern was seen in 120 cases (20%). Mucosal infiltration was seen in 57 cases (9%). Vascular, nerve and fat infiltration were uncommon and seen in 38 (6%), 18 (3%) and 13 (2%) cases, respectively. Lymph node metastasis was rare and seen in only four cases (1%). Hemorrhagic and cystic changes were common and seen in 284 (46%) and 296 (48%) cases, respectively.
Immunohistochemical Features
In the immunohistochemical assay for 383 patients of 613, expression of CD117 and CD34 was observed in 95 and 80% of the 383 patients. α-Smooth muscle actin was expressed in 28% of the patients and S-100 was expressed in 16% of the patients. Positive immunostaining of desmin was observed in three patients (1%).
Outcome of Patients without Predictive Parameters of Malignancy
There were 293 patients without any predictive parameters of malignancy. These patients were followed up for at least 1 year (mean 4.8 years, ranging from 1 to 31.5 years). Among them, 117 patients were followed up over 5 years, and the longest follow-up time was 31.5 years. There were no death and only three patients developed local relapse. One patient with 2.5 cm duodenal GIST developed recurrence 3.84 years after first enucleation, which was located 2 cm away from the primary site according to surgical record. The patient was then treated by duodenopancreatectomy. The second and the third patient with rectal GIST developed local relapse 5.4 and 9.0 years after excision and subsequently treated with enucleations. All of the recurrent tumors did not present any of the predictive parameters of malignancy. The disease-free survival and overall survival for these patients are listed in Table 1.
Outcome of Patients with Predictive Parameters of Malignancy
There were 320 patients with predictive parameters of malignancy. For disease-free survival, 297 patients were selected for further analysis after excluding 23 patients with palliative resection. Of the 297 patients, 151 patients developed recurrences and the estimated 5-year disease-free survival rate was 44% (mean 6.7 years, median 4 years, ranging from 0.2 to 17.4 years). Of the 151 patients developed recurrences, 63 developed abdominal or pelvic recurrences, 50 had liver metastases without recurrence in primary site, 30 had abdominal or pelvic recurrences and liver metastases, 7 developed local recurrence and 1 had metastasis at unknown location. For overall survival, 269 patients were selected for further analysis after excluding 51 patients treated with imatinib therapy. A total of 93 patients died of GISTs. The estimated 5-year overall survival rate was 60% (mean 9.3 years, median 8.0 years, ranging from 0.2 to 17.4 years).
Evaluation of the Stage and Grade of Patients with 12 Predictive Parameters of Malignancy
A total of 12 predictive parameters of malignancy were used. For a single patient, at least one and at most six predictive parameters of malignancy were identified in 318 patients and 2 patients had seven parameters. Table 1 showed follow-up data by tumor groups defined by the number of parameters. The disease-free survival and overall survival decreased with the increase of the number of parameters in patients without gross spread (or organ confined), but not in patients with gross spread.
Disease-free survival and overall survival were associated with the number of the predictive parameters of malignancy in patients without gross spread (P<0.0001 for both disease-free survival and overall survival), but not in patients with gross spread (P=0.882 and 0.441) (Table 2).
Furthermore, in patients without gross spread (organ confined), disease-free survival exhibited significant difference between patients with consecutive number of parameters, like 1 and 2, 2 and 3, 3 and 4, 4 and 5, and 5 and 6 (P=0.0390, 0.0340, 0.0120, 0.0381, 0.0225, respectively). For overall survival correlation, part of the pair of consecutive numbers exhibited significant difference. The correlation coefficients between 1 and 2, 2 and 3, 3 and 4, 4 and 5, and 5 and 6 were 0.1890, 0.0367, 0.0921, 0.0502, 0.7471, respectively (Table 2).
However, when some of the consecutive number groups were combined together, both disease-free survival and overall survival showed significance difference. For example, when group with 1+2 parameters vs 3+4 parameters and 3+4 vs 5+6 were compared, P<0.0001 was obtained for disease-free survival. P<0.0001 and 0.0033 were obtained for 1+2 vs 3+4 and 3+4 vs 5+6, respectively, for overall survival (Table 2). In addition, the disease-free survival showed significant difference between patients with and without microscopic spread (P=0.0009) (Table 2).
On the basis of the above results, we therefore divided GISTs with predictive parameters of malignancy into clinical stage I (or organ confined) and II based on the absence or presence of gross spread at the time of diagnosis, and clinical stage I was divided into pathological stage I and II based on the absence or presence of microscopic spread.
The degree of malignancy of patients in clinical stage I could be graded according to the number of malignant parameters and patients were classified into low (patients with one and two parameters), moderate (patients with three and four parameters) and high (patients with five and six parameters) degree of malignancy. Patients in clinical stage II were of the highest degree of malignancy regardless the number of parameters, and there was no need to grade patients with gross spread further.
Correlation of Prognosis with Clinical and Pathological Parameters, and Tumor Nature, Grade and Stage
First, we showed that most of the clinical and pathological parameters affected the prognosis (disease-free survival) of patients with GIST by univariate analysis when all of the patients with GIST were regarded as one group. A total of 11 clinical and 12 pathological parameters associated with adverse prognosis are listed in Table 3.
Second, the correlation of clinical and pathological parameters with prognosis was changed or lost when tumor nature (malignant or nonmalignant) was classified. For example, in 293 patients with nonmalignant GIST, only rectal GIST associated with adverse prognosis since 2 patients developed local recurrence in 28 patients with rectal GIST and 1 patient developed local recurrence in 265 patients with non-rectal GIST; in malignant group, there were seven clinical and seven pathological parameters associated with adverse prognosis (Table 3).
Third, when these 14 parameters were further analyzed by multivariate analyses, it revealed that 4 clinical and 5 pathological parameters were independent prognostic factors. The clinical parameters were adhesion (P=0.001), tumor rupture (P=0.006), gross spread (P=0.008) and anorectal GISTs (P=0.003); the pathological parameters were severe nuclear atypia (P=0.043), mitoses ≥10 per 50 high-power field (P<0.0001), coagulative necrosis (P<0.0001), vascular infiltration (P<0.0001) and fat infiltration (P=0.014). The tumor size had no prognostic function in these multivariate analyses system (Table 4).
However, when two stages and three-tier grade system were classified (Table 3), only a small number of parameters were associated with prognosis in different grades and stages, and the parameters were not consistent. Furthermore, tumor grade and stage as classified by our new system were strongly associated with disease-free survival independent of the clinical (Table 5) and pathological parameters (Table 6). The Kaplan–Meier plots of disease-free survival and overall survival are shown in Figures 9 and 10 for patients with nonmalignant GIST and malignant GIST including low, moderate, high grade in clinical stage I and patients in clinical stage II (P<0.0001 for both). The mean and median time of disease-free survival for these groups was 18.1 years, not reached; 10.7 years, 14.4 years; 4.8 years, 3.0 years; 1.7 years, 1.2 years and 1.3 years, 0.8 years, respectively. Patients with nonmalignant GIST could be cured by surgical resection alone. Therefore, there is no mean and median time for overall survival in these patients. The mean and median time of overall survival for the malignant patients including low, moderate, high grade in clinical stage I and patients in clinical stage II were 14.1 years, 15.6 years; 7.2 years, 6.8 years; 3.8 years, 3.0 years; 1.9 years, 1.2 years, respectively.
Relationship of Tumor Nature, Grade and Stage with the NIH Consensus Criteria
When the 613 patients with GIST were classified into different risk levels according to the NIH consensus criteria, 81 cases were classified into the very-low-risk level, 179 into the low-risk level, 98 into the intermediate-risk level, and 255 into the high-risk level. There were 5, 10, 14 and 125 patients who developed recurrence in the four risk levels. Among the lower three levels, only marginal difference was presented (P=0.06) for disease-free survival (Figure 11); for the high-risk level, patients could be classified further into three subgroups based on the NIH criteria: tumor size >5 cm and mitoses >5/50 per high-power field, tumor size >10 cm with any mitoses and mitoses >10 per 50 high-power field with any size. Among these subgroups, significant difference (P<0.0001) was presented for disease-free survival (Figure 12).
The distributions of all patients with GIST in various groups classified by the NIH system and by our current tumor nature, grade and stage classification system are shown in Table 7. There were 3 (1.0%) patients with recurrences in the nonmalignant group, 34 (25%) in the low-grade group, 65 in the moderate-grade group (63%), 36 (86%) in the high-grade group, and all of the 16 patients in clinical stage II treated with surgical resection with curative intention developed recurrence (100%). For each risk level stratified by the NIH criteria, there is a significant correlation of disease-free survival with our current classification system (all P's <0.0001). However, for each group stratified by our grading and staging system, there is no significant correlation of disease-free survival with the different risk levels classified by the NIH criteria, except for low-grade group (P=0.001).
Discussion
Tremendous progress has been made in the diagnosis and treatment of GIST since Hirota et al reported the molecular basis of GISTs. Most GISTs have a mutation in the c-kit proto-oncogene, leading to the constitutive expression of KIT protein. In addition, an activating mutation in the PDGFRA gene was identified in about 5% of GISTs.32 GISTs have a wide spectrum of clinical behavior that spans from indolent and curable lesions by surgical resection alone to highly malignant diseases that metastasize and become lethal.33 The treatment of GIST is complete excision when possible, and treatment with KIT/PDGFRA tyrosine kinase inhibitors, such as imatinib, when the tumor is unresectable or in metastatic stage. Unfortunately, tumor resistance to imatinib treatment is a significant problem, and the long-term success is limited. The imatinib resistance is usually generated by the development of the secondary mutations in the KIT or PDGFRA tyrosine kinase domains.4, 34, 35, 36 Although the tyrosine kinase inhibitor SU11248 and PKC412 can be used as the second-line treatments and some patients with imatinib-resistant GIST showed response to these drugs,37 the median time to disease re-progression was only about 6 months. Because imatinib adjuvant as well as neoadjuvant treatment can dramatically improve the prognosis for high-grade malignant GISTs,9 there are potential benefits to apply imatinib therapy preoperatively or postoperatively in patients with malignant GIST. Up to now, there is still no consensus on the selection of candidates for adjuvant therapy, mainly because the criteria predicting patients with a high risk of recurrence after the surgical removal of primary GISTs have yet to be established.
Initially it is proposed that the presence of genetic mutations could be used as a marker to predict malignancy or poor prognosis.38, 39, 40, 41, 42, 43 Since approximately 90% of the GISTs contain either KIT or PDGFRA gene mutations, it is apparently not a reliable criterion.44 Studies indicated that the type of mutation may predict prognosis.40, 41, 45, 46, 47 On the basis of our unpublished data and recent reports, the most reliable mutation type with independent prognostic role was homogenous deletion in exon 11 of KIT gene.48 However, the frequency of this homogenous mutation is rare. DNA ploidy, telomerase activity, immunohistochemical staining for cell proliferation antigens and other factors involved in the regulation of the cell cycle (p53, p16, p21, bcl-2, CD44, cox-2) have been investigated as prognostic factors, but no consistent results were obtained.49
It is known that GISTs exhibited a broad range of biologic behaviors. There is a strong need for a practical and reliable classification system that can be used to predict the clinical course of GIST patients and facilitate the design of treatment regimen. On the basis of previous investigations and our preliminary work,10, 11, 12, 13, 14, 15, 16, 17, 18 we collected the pathological and clinical data of a large number of GIST patients and assessed a simple and new grading and staging system. Patients from multiple hospitals were analyzed because it is expected to be more likely free of selection bias, which could occur in different hospitals or treatment trials.
We used 12 clinical and pathological predictive parameters of malignancy, and these predictive parameters of malignancy were divided into three types according to the distance of spread from the primary sites. Gross spread (clinical stage II) parameters included liver metastasis and peritoneal dissemination; microscopic spread (pathological stage II) parameters included lymph node metastasis, vascular, fat, nerve and mucosal infiltration; and localized (pathological stage I) parameters included mitotic count ≥10 per 50 high-power fields, muscularis propria infiltration, coagulative necrosis, perivascular pattern and severe nuclear atypia.
The malignant and nonmalignant tumors were separated first by the presence and absence of any of these parameters; second, the clinical stage II and stage I were classified based on presence and absence any of gross spread parameters; and finally the natural tiers or combined tiers of malignant tumors were represented by the number of parameters that were identified for the tumor. When the tumors were staged and graded by the new system, strong correlations of disease-free survival and overall survival with the tumor stage and grade were obtained. This is the first time that the degree of malignancy was demonstrated by the number of the predictive parameters of malignancy.
Previous studies have demonstrated that the adverse outcome of GISTs correlates with the increase in the tumor size,12, 16, 25, 50, 51, 52, 53 mitotic rate,20, 25, 51, 52, 54 cellularity51, 52, 55 and nuclear atypia.16 In addition, tumor site23, 56, 57 from the upper to lower GI tract seemingly had an effect on the outcome too. The similar observations were made in our study. When all patients were treated as one group, each pathological parameter and most of the clinical parameters showed effect on the disease-free survival in a way as previously reported. However, when patients were grouped into more homogenous groups by nonmalignancy, malignant degrees and stages, less and less clinical and pathological factors had significant correlation with disease-free survival by univariate analysis. For example, only one parameter correlated with disease-free survival in nonmalignant GISTs, four correlated with disease-free survival in low-grade tumors, three in moderate-grade tumors, one in high-grade tumors and three in clinical stage II tumors. Moreover, these parameters are not consistent in different stage and grade of tumors. In addition, tumor size no longer showed any prognostic function in multivariate analyses.
On the contrary, when tumors were graded and staged by our system with the identified parameters, the disease-free survival nearly always strongly correlated with the malignant grade, indicating that the clinical stage and grade were closely associated with the prognosis of GISTs. On the basis of our analytic method and results, it can be concluded that the predictive role of a single predictive parameter of prognosis varied with the tumor nature, grade and stage. Therefore, the role of a single parameter is limited in prognosis prediction.
The NIH consensus criteria based on the tumor size and mitotic count have gained general acceptance to predict the prognosis of GISTs. By comparing the NIH consensus criteria with our staging and grading system, interesting differences were observed. We found our predictive parameters of malignancy showed malignant course for some patients with small and mitotically inactive GISTs, for whom the NIH consensus criteria only predict low risk. Moreover, some patients with GISTs of bigger size did not show any of the predictive parameters of malignancy experienced indolent outcome, for whom the NIH consensus criteria should predict high risk. These observations indicated that tumor size/mitoses alone are not adequate for the accurate prediction of prognosis. In addition, on the one hand, we found only marginal significant difference among the very low level, low level and intermediate level, indicating that these levels could be combined together; on the other hand, we found significant difference existed in the subgroups of the high-risk level, indicating that these subgroup should be stratified further, especially for mitoses >10 per 50 high-power field with any size. These problems of the grading system in the NIH criteria were also pointed out by Huang et al,58 and sometimes existed in other soft sarcomas.59
In conclusion, we showed that the stage and grade of tumors classified by our 12 clinical and pathological parameters strongly correlated with the prognosis of GISTs. Nonmalignant and malignant tumors were first identified, the stage was second classified and the number of predictive parameters of malignancy was used to represent the degree of malignancy in organ-confined sarcoma. The prognosis of malignant GISTs was strongly associated with tumor stage and grade. Initial comparison showed that our system can predict the prognosis better than the NIH consensus criteria. For nonmalignant patients, it is unnecessary for further therapy, for patients with malignant GIST especially higher degree of malignancy, adjuvant therapy should be recommended postoperatively. The usefulness of our new staging and grading system in predicting the outcome of imatinib therapy will be studied in future randomized trials with imatinib as a neoadjuvant or adjuvant treatment for GISTs.
References
Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577–580.
Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 2003;125:660–667.
Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052–1056.
Chen LL, Trent JC, Wu EF, et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res 2004;64:5913–5919.
Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620–625.
Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626–632.
Salazar M, Barata A, Andre S, et al. First report of a complete pathological response of a pelvic GIST treated with imatinib as neoadjuvant therapy. Gut 2006;55:585–586.
Haller F, Detken S, Schulten HJ, et al. Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann Surg Oncol 2007;14:526–532.
Andtbacka RH, Ng CS, Scaife CL, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol 2007;14:14–24.
Akwari OE, Dozois RR, Weiland LH, et al. Leiomyosarcoma of the small and large bowel. Cancer 1978;42:1375–1384.
Shiu MH, Farr GH, Papachristou DN, et al. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer 1982;49:177–187.
McGrath PC, Neifeld JP, Lawrence Jr W, et al. Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg 1987;206:706–710.
Hasegawa T, Matsuno Y, Shimoda T, et al. Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol 2002;33:669–676.
Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002;20:3898–3905.
Shirin H, Kravtsov V, Shahmurov M, et al. The cyclin-dependent kinase inhibitor, p27, has no correlation with the malignant potential of GIST. Digestion 2007;75:4–9.
Ng EH, Pollock RE, Munsell MF, et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992;215:68–77.
Conlon KC, Casper ES, Brennan MF . Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Oncol 1995;2:26–31.
Gutierrez JC, De Oliveira LO, Perez EA, et al. Optimizing diagnosis, staging, and management of gastrointestinal stromal tumors. J Am Coll Surg 2007;205:479–491.
Hou YZX, Lu S, Zhou Y, et al. Study on malignancy, staging and grading for gastrointestinal stromal tumors. The 44th ASCO Annual Meeting. Chicago, 2008.
Trupiano JK, Stewart RE, Misick C, et al. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol 2002;26:705–714.
Yan H, Marchettini P, Acherman YI, et al. Prognostic assessment of gastrointestinal stromal tumor. Am J Clin Oncol 2003;26:221–228.
Miettinen M, Sobin LH, Lasota J . Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52–68.
Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477–489.
Miettinen M, Sarlomo-Rikala M, Sobin LH, et al. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol 2000;24:211–222.
Koga H, Ochiai A, Nakanishi Y, et al. Reevaluation of prognostic factors in gastric leiomyosarcoma. Am J Gastroenterol 1995;90:1307–1312.
Fujimoto Y, Nakanishi Y, Yoshimura K, et al. Clinicopathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer 2003;6:39–48.
Tworek JA, Goldblum JR, Weiss SW, et al. Stromal tumors of the abdominal colon: a clinicopathologic study of 20 cases. Am J Surg Pathol 1999;23:937–945.
Tworek JA, Goldblum JR, Weiss SW, et al. Stromal tumors of the anorectum: a clinicopathologic study of 22 cases. Am J Surg Pathol 1999;23:946–954.
Miettinen M, Sarlomo-Rikala M, Sobin LH, et al. Gastrointestinal stromal tumors and leiomyosarcomas in the colon: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases. Am J Surg Pathol 2000;24:1339–1352.
Miettinen M, Furlong M, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol 2001;25:1121–1133.
Cooper PN, Quirke P, Hardy GJ, et al. A flow cytometric, clinical, and histological study of stromal neoplasms of the gastrointestinal tract. Am J Surg Pathol 1992;16:163–170.
Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708–710.
Miettinen M, Sarlomo-Rikala M, Lasota J . Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999;30:1213–1220.
Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 2006;24:4764–4774.
Tamborini E, Bonadiman L, Greco A, et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology 2004;127:294–299.
Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005;11:4182–4190.
Chow LQ, Eckhardt SG . Sunitinib: from rational design to clinical efficacy. J Clin Oncol 2007;25:884–896.
Andersson J, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology 2006;130:1573–1581.
Ernst SI, Hubbs AE, Przygodzki RM, et al. KIT mutation portends poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest 1998;78:1633–1636.
Lasota J, Jasinski M, Sarlomo-Rikala M, et al. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol 1999;154:53–60.
Martin J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 2005;23:6190–6198.
Taniguchi M, Nishida T, Hirota S, et al. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res 1999;59:4297–4300.
Wardelmann E, Losen I, Hans V, et al. Deletion of Trp-557 and Lys-558 in the juxtamembrane domain of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. Int J Cancer 2003;106:887–895.
Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 2001;61:8118–8121.
Lasota J, Kopczynski J, Sarlomo-Rikala M, et al. KIT 1530ins6 mutation defines a subset of predominantly malignant gastrointestinal stromal tumors of intestinal origin. Hum Pathol 2003;34:1306–1312.
Lasota J, Dansonka-Mieszkowska A, Stachura T, et al. Gastrointestinal stromal tumors with internal tandem duplications in 3′ end of KIT juxtamembrane domain occur predominantly in stomach and generally seem to have a favorable course. Mod Pathol 2003;16:1257–1264.
Lasota J, Dansonka-Mieszkowska A, Sobin LH, et al. A great majority of GISTs with PDGFRA mutations represent gastric tumors of low or no malignant potential. Lab Invest 2004;84:874–883.
Lasota J, vel Dobosz AJ, Wasag B, et al. Presence of homozygous KIT exon 11 mutations is strongly associated with malignant clinical behavior in gastrointestinal stromal tumors. Lab Invest 2007;87:1029–1041.
Bruck R, Arber N . Prognostic markers in gastrointestinal stromal tumors—we are not there yet. Digestion 2007;75:1–3.
Kimura H, Yonemura Y, Kadoya N, et al. Prognostic factors in primary gastrointestinal leiomyosarcoma: a retrospective study. World J Surg 1991;15:771–776.
Ueyama T, Guo KJ, Hashimoto H, et al. A clinicopathologic and immunohistochemical study of gastrointestinal stromal tumors. Cancer 1992;69:947–955.
Tazawa K, Tsukada K, Makuuchi H, et al. An immunohistochemical and clinicopathological study of gastrointestinal stromal tumors. Pathol Int 1999;49:786–798.
DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51–58.
Evans HL . Smooth muscle tumors of the gastrointestinal tract. A study of 56 cases followed for a minimum of 10 years. Cancer 1985;56:2242–2250.
Franquemont DW, Frierson Jr HF . Muscle differentiation and clinicopathologic features of gastrointestinal stromal tumors. Am J Surg Pathol 1992;16:947–954.
Miettinen M, Lasota J . Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466–1478.
Miettinen M, Lasota J . Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
Huang HY, Li CF, Huang WW, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery 2007;141:748–756.
Deyrup AT, Weiss SW . Grading of soft tissue sarcomas: the challenge of providing precise information in an imprecise world. Histopathology 2006;48:42–50.
Acknowledgements
This study was performed under the auspices of National Natural Science Foundation of China (no. 30300152) and Shanghai Science Technique Planning Foundation (no. 064119622).
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
All authors read and approved the final paper. The opinions contained herein are the expressed views of the authors. We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hou, YY., Lu, SH., Zhou, Y. et al. Stage and histological grade of gastrointestinal stromal tumors based on a new approach are strongly associated with clinical behaviors. Mod Pathol 22, 556–569 (2009). https://doi.org/10.1038/modpathol.2009.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2009.11
Keywords
This article is cited by
-
Gastrointestinal stromal tumors (GISTs) with remarkable cystic change: a specific subtype of GISTs with relatively indolent behaviors and favorable prognoses
Journal of Cancer Research and Clinical Oncology (2019)
-
Clinicopathological Features and Prognosis of Small Gastric Gastrointestinal Stromal Tumors (GISTs)
Journal of Gastrointestinal Surgery (2019)