Abstract
BRAFV600E mutation has been frequently reported in different types of melanocytic lesions, but its role in melanomagenesis is poorly understood, having been associated with either the proliferative-induced MAPK pathway activation or the acquisition of oncogene-driven senescence. The presence of BRAF alterations has been related to sun exposure, although the molecular mechanisms underlying this event are only partly known. To elucidate the relationships among BRAF/NRAS alterations, MAPK pathway activation, and sun exposure, we examined 22 acquired nevi and 18 cutaneus melanomas from 38 patients. Microdissected tissues from each lesion were subjected to BRAF/NRAS mutation analysis by sequencing, allele-specific PCR and pyrosequencing assay. The same lesions were also examined for the expression of phosphorylated ERK1/2. Phototype and an accurate history of sun exposure were evaluated for each patient. BRAFV600E mutation was detected in 50% of the acquired nevi and in 70% of the cutaneus melanomas in the absence of NRAS alterations. The fraction of alleles carrying BRAFV600E substitution was variable but strongly associated with sun exposure. In contrast, no relationship was evidenced between the presence of this mutation and patients' phototype, phosphorylated ERK1/2 expression, or Clark's level. Our findings indicate that in melanocytic lesions, BRAFV600E mutation can affect a subset of the cells and is associated with the type and quantity of sun exposure. This mutation is independent of the nevo-melanoma progression and unrelated to ERK phosphorylation, suggesting that alternative mechanisms to the MAPK activation are also involved in this type of transformation.
Similar content being viewed by others
Main
Cutaneus melanoma can be regarded as the consequence of the loss of controlling genetic mechanisms by disrupting initiating events due to environmental factors.
Somatic genetic alterations affecting the mitogen-activated protein kinase (MAPK) signaling pathway are believed to be determinant for the onset of this type of neoplasia. Among the genes controlling the MAPK pathway, BRAF has been found highly mutated in a variable percentage of nevi and melanomas.1, 2 However, at present, the exact contribution of these frequent mutations to the initiation and progression of the melanocytic lesions is still poorly understood. Most (>90%) of the reported BRAF alterations are oncogenic mutations, located in the region coding for the kinase domain. These alterations are prevalently due to T1799A transversion leading to a substitution of valine by glutamic acid at codon 600 (V600E). BRAFV600E mutations have been shown to occur early during the progression from nevus to melanoma,3, 4 but the association of this alterations with prognosis is controversial.5, 6, 7 Recent reports have also pointed out that the frequency of this oncogenic mutation varies remarkably according to the type of melanocytic lesions,8, 9, 10 being the common acquired nevi and the cutaneus melanomas among the more affected ones. Alterations involving NRAS, another gene regulating the MAPK signaling pathway, have also been associated with melanomagenesis, although to a lesser extent. Mutations affecting this gene occur prevalently at codon 61 (NRASQ61) and have been reported in up to 18–25% of common acquired nevi and cutaneus melanomas.8, 11 Generally, activating mutations of BRAF and NRAS oncogenes are mutually exclusive. Similar to BRAF mutations, NRAS alterations have been found associated with the initiation of melanomagenesis, but not with the progression of the lesion or patient's survival.3, 11, 12
Both BRAF and NRAS oncogenic mutations are expected to activate the MAPK signaling pathway.13 However, in melanomagenesis, this evidence is controversial, as some studies performed on different types of melanocytic lesions have shown that the MAPK pathway activation can be achieved independently of BRAF or NRAS alterations.14 In addition, there are in vitro data supporting the involvement of BRAFV600E mutation in the induction of cell cycle arrest and senescence of nevocytes.15
Among the environmental factors related to the onset of cutaneus melanomas, exposure to sunlight has been generally accepted to have a major role.16 Epidemiological data have shown that the type of exposure is determinant to the melanocytic transformation process, an intense intermittent sun exposure during childhood being particularly deleterious.17 In addition to UVB, exposure to UVA is also now recognized to have a role in the development of melanoma. However, the molecular mechanisms by which UVB/UVA operate on cellular target genes are known only partly.18 Some reports have shown a relationship between the type of sun exposure and the alterations affecting the BRAF–RAS pathway.19, 20, 21 A higher frequency of BRAF mutations has been found in cutaneus melanomas arising in intermittent sun-exposed sites with respect to the lesions developing in mucosal membranes or unexposed sites.22 On the contrary, RAS alterations are more common in skin sites subjected to chronic sun exposure. In a study carried out on 126 melanomas exposed to a variable degree of sunlight, Curtin et al23 showed that the type of sun exposure activates distinct genetic pathway. Nevertheless, the relationship between sun exposure and BRAF mutation has been investigated mainly in melanomas, whereas it is almost unknown in nevi.
In the present report, we looked for the relationships among the presence of BRAF/NRAS alterations, the MAPK pathway activation, and sun exposure in 22 common nevi and 18 cutaneus melanomas, including three melanomas with underlying nevi. To this aim, data from an accurate BRAF/NRAS mutation analysis and the immunohistochemical assessment of phosphorylated ERK1/2 were associated with skin phototypes and detailed information about the history of sun exposure.
Tumor specimens, patients and methods
Tumor Specimens and Clinical Data
We collected archivial, paraffin-embedded tissue samples from 22 common acquired melanocytic nevi (3 junctional, 13 compound, and 6 dermal) and 18 primary cutaneus melanomas (two Clark's level II, four level III, six level IV, and two level V). The 22 common acquired nevi were obtained from 20 Italian patients (14 women and 6 men; median age: 40.6 years). Nevi N22A and N22B were excised from distant sites of the same patient, lumbar region and back, respectively. Similarly, N27A and N27B were removed from the neck and the hip of a second patient. Melanomas were obtained from 18 Italian patients (7 women and 11 men; median age: 55.4 years). M2, M3, and M10 were complex lesions in which melanoma and residual nevus were histologically identifiable. Clinical data of patients are reported on Tables 2 and 3.
After having obtained informed consent, the dermatologists (AZ and FP) interviewed the patients by using a standardized written questionnaire to collect clinical data and medical history. Some of the questions were focused on the quantity and quality of sun exposure that patients had undergone during their lifetime. These parameters were considered to give each case a numerical value. In-depth evaluation was made for cases of sunburn during childhood and adulthood, type of exposure during the day, length of exposure times, use of sunscreens, and the use of sun lamps (if extensive). The resulting scores are summarized in Table 1a. The phototype of the patients under study was also taken into consideration. Table 1b shows a classification of sun-reactive skin types according to Fitzpatrick.24
All melanomas were excised from skin areas that had been subjected to intermittent sun exposure. Among nevi, six cases (N1, N16, N21, N25, N28, and N31) were excised from unexposed skin areas. These nevi were given a value of 0 (Table 2).
This study was approved by the medical ethical committee of the IRCC, and carried out according to the principles of the Declaration of Helsinki.
Microdissection and DNA Extraction
Paraffin-embedded H&E-stained tissue sections from the patients were histologically evaluated for the presence of different components of the melanocytic lesion and contaminating normal cells (lymphocytes). A percentage of nevus-melanoma cells was assigned to each lesion. According to this percentage, nevi and melanomas were subjected to microdissection by means of optical microscope or laser microdissector (Leica AS-LMS, Leica Microsystems, Wetzlar, Germany). DNA extraction was performed as follows: cells were collected in Eppendorf tubes, deparafinized by xylol, rehydrated and resuspended in 250 μl of lysis buffer containing 100 mM Tris-HCl (pH 8.5), 5 mM EDTA, 200 mMNaCl, 2% SDS, and 15 μl of proteinase K (20 mg/ml). After incubation for 1 day at 55°C, samples were precipitated with isopropanol, washed with 70% ethanol. According to the quantity of pellets, DNAs were resuspended in 30–50 μl of 10 mM Tris-HCl (pH 8.5).
Mutation Analysis
Sequencing and allele-specific PCR
Sequence analysis was based on BRAF and NRAS sequences (GenBank accession number NM_00433 and NM_002524 for BRAF and NRAS, respectively). BRAF exons 11 and 15 were subjected to PCR amplifications using primers as previously reported.10 As far as NRAS exons 1 and 2 are concerned, we used the following primers: exon 1, forward 5′-TAAGGATGGGGGTTGCTAGA-3′ and reverse 5′-TGCATAACTGAATGTATACCCAAAA-3′; exon 2, forward 5′-TTGCATTCCCTGTGGTTTTT-3′ and reverse 5′-TGGTAACCTCATTTCCCCATA-3′. Briefly, for all exons, thermal cycling was carried out in a final volume of 50 μl containing 100 μM each dNTP, 10 pmole of primers, 3 μl of DNA extract and 5 U of Taq polymerase (Thermoprime Plus DNA Polymerase, Abgene, Epsom, UK). The amplification protocol that we used consisted of an initial denaturation of 4 min followed by 40 cycles with denaturation at 94°C for 45 s, annealing at 55°C for 45 s and extension at 72°C for 1 min and 30 s. PCR products were purified by MinElute Gel Extraction Kit (Qiagen, Hilden, Germany) and directly sequenced using ALF express sequencer and Thermo Sequenase Cy5.5 Dye Terminator Cycle Sequencing Kit (GE Healthcare, Little Chalfont, UK). A second round of sequence analysis was carried out on PCR products purified with the use of ExoSAP-IT (USB Corporation, Cleveland, OH, USA) using 3730 DNA Analyzer and Big Dye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). All lesions that resulted to be wild type for codon 600 were further analyzed by allele-specific PCR. To this aim, two different forward primers with substitution of a single base at the end of the primer (5′-GTGATTTTGGTCTAGCTACAGT-3′ and 5′-GTGATTTTGGTCTAGCTACAGA-3′) were designed to amplify the wild-type allele or BRAFT1799A mutation, respectively; in both cases, the same reverse primer was used (5′-GGCCAAAATTTAATCAGTGGA-3′). DNAs were amplified with the two sets of primers on the following conditions: denaturation for 2 min at 94°C, 25 cycles at 94°C for 30 s, 62°C for 30 s and 72°C for 30 s, and a final elongation for 7 min at 72°C. PCR were carried out with two wild-type controls and a BRAFT1799A mutated sample used at different dilutions.
Mutation quantification
In the BRAFV600E lesions, we assessed the percentage of alleles carrying the mutation by pyrosequencing analysis, as previously reported by Edlundh-Rose et al.25 To perform this assay, which is based on ‘sequencing by synthesis’ principle, PCR amplifications were carried out with the following primers: 5′-biotin-CTTCATAATGCTTGCTCTGATAGG-3′ (forward) and 5′-GCATCTCAGGGCCAAAAA-3′ (reverse); samples were denaturated at 94°C for 5 min, amplified for 35 cycles consisting of 94°C for 30 s, 58°C for 30 s, and 72°C for 45 s and elongated at 72°C for 7 min. The 5′-biotinylated PCR products of the region including codon 1799 were immobilized onto streptavidin-coated paramagnetic beads (GE Healthcare), denaturated by 0.1 mol/l NaOH and released according to the manufacturer's instructions using PyroMark Vacuum Prep Workstation (Biotage, Uppsala, Sweden). These reactions were performed in a 96-well plate using Pyro Gold Reagents (Biotage). The primed single-stranded DNA templates were subjected to real-time sequencing of the region including codon 600 by using the reverse primer 5′-CCACTCCATCGAGATT-3′. A titration series with different dilutions of a sample containing the V600E mutation was set up to evaluate the linear correlation between the height of the peaks and the percentage of mutant alleles in the heterozygous lesions. These percentages were calculated by using PSQ96MA software (Biotage) for allelic quantification.
Phosphorylated ERK1/2 Immunohistochemistry
The MAPK pathway activation was evaluated using an antibody to dually phosphorylated ERK1/2 protein on tissue sections. To this aim, Anti p44/42 MAPK (Thr202/Tyr204) mouse mAb was obtained from Cell Signaling Technology Inc. (Beverly, MA, USA). Briefly, 3 μm formalin-fixed paraffin-embedded tissue sections were rehydrated, pretreated with 1:10 dilution Target Retrieval Solution High pH (Dako Cytomation, Carpinteria, CA, USA) for 15 min in microwave oven at 600 W and blocked in H2O2 for 5 min. Primary anti-p44/42 at 1:400 dilution was incubated for 1 h at room temperature and detected using IgG anti-mouse/rabbit-poly-HRP (Novocastra, Newcaste Upon Tyne, UK) for 30 min and visualized with DAB Chromogen. As active ERK1/2 localizes in the nucleus, but can also exhibit cytoplasmic targets, both cytoplasmic and nuclear immunostaining were considered, and the overall score was assessed as following: absent or weak, + (20% positive cells, low expression); medium, ++ (25–50% positive cells, moderate expression); and strong, +++ (>50% positive cells, high expression). As previously reported by Saldanha et al,8 the endothelium of peritumoral vessel was used as an internal positive control.
Statistical Analysis
The χ2 test and Fisher's exact test (when appropriate) were used to infer proportions when assessing the presence of association between BRAF mutation, type of lesion, Clark's level, immunohistochemistry, exposure sites, phototype, and sun exposure. To avoid ignoring the available information on the ordinal nature of the variables, tests based on rank correlation coefficients (Spearman and Kendall's τ) were used. Student's t-test and Wilcoxon rank-sum test were also carried out for phototype and sun exposure. P<0.05 was considered significant. All calculations were done in R statistical programming language (http://www.R-project.org).
Results
BRAF/NRAS Mutations
BRAF mutations were detected in 11 of the 22 (50%) common nevi and in 13 of the 18 (70%) cutaneus melanomas. All the alterations were due to GTG to GAG transversion on nucleotide 1799 (T1799A). This substitution leads to the oncogenic change V600E. No mutation was found in exon 11 of BRAF gene as well as in exons 1 and 2 of NRAS gene.
No further mutations were evidenced when the wild-type lesions were rechecked for the presence of T1799A by allele-specific PCR.
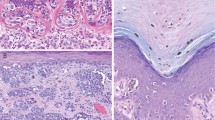
The sequencing analysis showed a variable level of the heights of the T/A peaks among the nevi and melanomas exhibiting BRAFV600E. These different levels were suggestive of the presence of a variable number of alleles with this mutation (Figure 1). To assess this evidence, all samples were analyzed by pyrosequencing. Supporting the sequencing data, this method showed that the microdissected mutant lesions of both nevi and melanomas were characterized by different percentages of T1799A alleles, with a range included between 5 and 40%. In addition, we found that pyrosequencing detected less than 25% of BRAFV600E alleles when the mutant peak of the corresponding sequences was lower than 50% of the wild-type signal (Figure 1b). The prevalence of the lesions characterized by less than 25% of BRAFV600E alleles was 36 and 40% among the nevi and melanomas, respectively (Tables 2 and 3).
The comparison between sequencing and pyrosequencing analysis of some lesions with different levels of BRAFV600E mutation (T1799A substitution). (a) A BRAF wild-type lesion; (b) the sequencing of a sample with the A peak lower than 50% of the T signal and its corresponding pyrogram characterized by the presence of <25% of the mutant alleles; (c and d) two lesions with the A signal higher than 50% and their pyrograms showing >25% of the mutant alleles. The pyrograms are reported in reverse.
The presence of BRAFV600E mutation was independent of the type of the microdissected lesion (P=0.63; Table 4a). For two patients (cases N22 and N27 in Table 2), we checked the nevi arisen in distant sites: BRAFV600E mutation was detected in all lesions. In the first subject, (N22) the T/A transversion was found in the two nevi obtained from the lumbar region and from the shoulder; in the second case, (N27) BRAF mutation was detected in the nevi excised from both the neck and the hip. We also analyzed three lesions with underlying nevi (cases M2, M3, and M10 in Table 3). In these tissues, either BRAF mutation was found in the nevus and melanoma (cases M2 and M10) or both lesions were wild type (case M3). The level of the T/A substitution was similar in the two-microdissected components of cases M2 and M10 (Table 3). In the case of melanoma M23, which was laser microdissected into in situ and invasive components, both tissues resulted to be wild type (Table 3). As far as melanomas are concerned, no relationship was evidenced between the presence of BRAFV600E mutation and Clark's level (P=1, Table 4c).
Phosphorylated ERK1/2 Immunohistochemistry
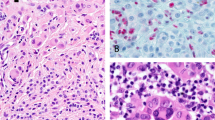
No correlation was evidenced between the presence of BRAFV600E mutation and the expression of phosphorylated ERK1/2 (P=0.72, Table 4a). This lack of association was found in the common nevi as well as in the cutaneus melanomas (P=0.8 and P=1, Table 4b and c), and was independent of the level of ERK1/2 expression (P=0.5 and P=0.19, Table 4b and c). However, considerable differences in the staining of phosphorylated ERK1/2 were observed between the two types of lesion. In the nevi, a positive expression was detected in only six cases (N35, N29, N14, N16, N20, and N21) that showed a weak immunostaining in not more than 10–15% of the cells (score +, low expression) (Table 2). In two of these cases (N20 and N29), the ERK1/2 protein expression involved just 5% of the cells and was limited to the cytoplasmic compartment (Figure 2b). As a matter of fact, in 8 out of the 11 melanocytic nevi with BRAF mutation no positive expression of pERK1/2 was identified (Figure 2a). Interestingly, five of these lesions (N36, N23, N26, N27A, and N27B) were characterized by a higher level of mutant alleles (>25%) (Table 2). On the contrary, a considerable high expression of pERK1/2 was evidenced among melanomas consistent with a fully MAPK pathway activation (Figures 2c–f). In five of these lesions, about 50–90% of the cells were found positive for pERK1/2 immunostaining (score +++; high expression), whereas in other five melanomas about 20–40% cells (score ++; moderate expression) were positive for the expression of this phosphorylated protein. In all these cases, we evidenced a nuclear staining variably associated with cytoplasmic expression (Figures 2c and d). In two combined lesions of melanoma with an underlying nevus (cases M2 and M10), we found a considerable level of pERK1/2 expression in both the nevus and the melanoma components (40% positive cells for M2 and 25% for M10). However, in the nevus, the staining was prevalently cytoplasmic, whereas it became mostly nuclear in the melanoma component. The pERK1/2 staining was independent of the level of T1799A substitution (Table 3). Similarly, a high percentage of positive cells (about 70%) for pERK1/2 expression was detected in both in situ and invasive components of case M23 characterized by the absence of BRAFV600E mutation (Figures 2e and f). Finally, no correlation was found between phosphorylated ERK1/2 and Clark's level (P=0.28, Table 4c).
Phosphoprotein ERK1/2 immunohistochemistry. (a) Sections from nevus N23 negative for pERK1/2 staining; the same sample was characterized by >25% alleles carrying BRAFV600E mutation. (b) Lesion N20 showed 5% of the cells positive for pERK1/2 (weak expression, +); staining was mainly cytoplasmic; this nevus was provided with >25% mutant alleles. (c) Melanoma M18 exhibited 35% positive cells for ERK1/2 staining (medium expression ++); the staining was both cytoplasmic and nuclear; pyrosequencing analysis of this lesion evidenced >25% mutant alleles. (d) Melanoma M18 at different magnifications. (e) In situ component of melanoma M23. (f) Invasive component of melanoma M23; both in situ and invasive components of this lesion were characterized by the presence of 70% positive cells (high expression +++); the staining was found in cytoplasm as well as in nucleous; this lesion was wild type for BRAF/NRAS genes (magnification: a and b, × 10; c, × 20; d–f, × 40).
BRAF Mutations, Sun-Exposure and Phototype
We evidenced a strong association between the presence of mutation at codon 600 of BRAF and sun exposure, measured according to the score reported in Table 1 (P<0.0001, Table 4a). This association was highly significant in both nevi and melanomas (P=0.0011 and P=0.002, Table 4b and c). The strong association was confirmed when the lesions were taken into consideration according to the percentage of mutant alleles: wild type, <25 and >25% (P<0.0001, Table 4a). As far as nevi are concerned, we also found a significant correlation (P=0.02) between the presence of BRAFV600E and the nevi excised from sun-exposed skin with respect to those derived from unexposed sites (Table 4b). By contrast, in both the two types of lesions, the patients' phototype was not associated with the substitution at codon 600 (P=0.18, Table 4a).
Discussion
In this study, we detected the presence of BRAFV600E mutation in 50% of the common nevi and in 70% of the cutaneus melanoma.
Overall, our results evidenced that in both these types of lesions, BRAFV600E substitution can affect a variable fraction of the alleles. It has been evidenced that, in junctional and small compound nevi, direct sequencing fails to detect the presence of BRAFV600E when this affects less than 20% of the cells.26 By combining microdissection and an accurate mutation analysis, including allele-specific PCR and pyrosequencing, we were able to perform a sensitive analysis and to quantify the percentage of these mutant alleles.
After sequencing, an underestimation of the presence of BRAFV600E was ruled out in the wild-type lesions by performing allele-specific PCR analysis. In the mutant tissues, the quantification of T1799A allele was carried out by using pyrosequencing. This method has been proved to be a useful approach to quantitatively detect somatic mutations in genetic syndromes causing mosaicism27 and it has been recently applied to perform BRAF and NRAS genotyping in melanomas.28 In the present study, the percentage of alleles carrying T1799A ranged from 5 to 45%, suggesting that in some lesions only a fraction of the melanocytes are affected by this oncogenic substitution. However, even on homogeneous microdissected tissue, a direct association between the fractions of mutated alleles and cells cannot be claimed, as allelic imbalances due to chromosome 7q rearrangements could occur in melanomas. Moreover, although microdissection is a reliable and helpful method to separate different cell population in paraffin-embedded tissues, we cannot formally exclude the presence of contaminating non-melanocytic cells, particularly in nevi.
The percentage of alleles carrying BRAFV600E substitution was found to be independent of the type of lesion. This supports the hypothesis of BRAF mutations as precocious events that are preserved throughout progression. This evidence was pointed out by the analysis of the three melanomas with residual nevi. In these cases, alleles carrying BRAF mutation were found in both components or, alternatively, were absent in each part of these lesions. Similarly, in another melanoma sample of this set (M23), no onset of V600E was evidenced in the transition from in situ to vertical growth phase. A role for this mutation as an initiating event is further supported by the lack of correlation between the presence of T1799A substitution and the Clark's level, a histological measure of melanoma progression. Accordingly, Omholt et al3 showed that BRAF mutation can be detected equally in radial growth phase (RGP) and vertical growth phase (VGP) melanomas.
The fact that the percentage of mutated alleles does not increase in the nevus-melanoma transition implies that the acquisition of this mutation does not provide the cells with that proliferative advantage, which is necessary to overwhelm the remaining cell population. BRAFV600E mutation is expected to constitutively activate the RAF/MEK/ERK pathway conferring the cells a proliferative and invasive potential. Nevertheless, in melanomas, the role of this commonly acquired BRAF mutation in the activation of the MAPK pathway is controversial. It has been shown that in nevi, the initial moderate proliferation supported by BRAFV600E is followed by a cell cycle arrest, implying that this mutation is associated with an oncogene-driven senescence process.15 In accordance with previous reports,8, 14 we did not find any association between this substitution and the pERK1/2 immunohistochemical expression. This turned out to be clearly evident in nevi where only few cases showed a poor staining (5–10% positive cells), independently of the presence of BRAF mutation. Melanomas of our series generally displayed a stronger ERK1/2 expression (up to 50–70% positive cells), but this staining was not related to the presence of BRAFV600E or to the fraction of the cells carrying this transversion. These results suggest that a full MAPK activation is achieved only when the lesion is at the stage of melanoma, when several other events can contribute to the activation of the RAF/MEK/ERK pathway. An overexpression of wild-type BRAF is one of the mechanisms underlying the activation of the MAPK pathway.29 Alternatively, the MAPK signaling cascade can be modulated by the inhibition of MAPK phosphatases or by the suppression of RAF kinase inhibitors.30, 31 The phosphorylation of ERK can also be due to the interplay of different signaling events. Among these, in late-stage melanomas, there are an overexpression of EGFR,32 and/or an increase of autocrine FGF signaling.33
It is well-known that a history of sunburn and intermittent exposure to sunlight may promote the development of melanoma.17 In cutaneus melanomas, mutations of BRAF gene have been related to intermittent sun exposure.22 Taking advantage of this finding, we collected an accurate history of the quantity and quality of sun exposure that each patient had undergone. By using this approach, we showed a strong association between the sun exposure, expressed as a score, and the presence of BRAFV600E mutation. Thomas et al34 have recently shown that the occurrence of BRAF mutations is associated with the early life UV exposure. In the present study, we found that there is a quantitative relationship between the level of sun exposure and the percentage of the alleles carrying V600E substitution. Our findings evidenced that the type of sun exposure is determinant for the onset of BRAFV600E mutation not only in cutaneus melanomas of exposed skin but also in common nevi subjected to intermittent light exposure. Accordingly, we observed an absence of NRAS alterations that had been mostly reported in lesions excised from chronic sun-exposed sites. Interestingly, we found that the presence of this BRAF mutation is independent of the patient's phototype. Variant alleles of MC1R, the gene encoding the melanocortin-1 receptor, have been shown to contribute to the onset of BRAF mutations in sun-exposed melanomas.35 However, this event may be partly unrelated to pigmentation, which is an important feature of skin phototype. As a matter of fact, Tadokoro et al36 showed a relationship between melanin content and DNA damage induced by UV exposure.
BRAFV600E alteration does not show the typical UVB signature mutations, which are characterized, through cyclobutane pyrimidine dimers and 6–4 photoproducts, by C/T and CC/TT transitions. Taking into consideration different variables such as nearby potential pyrimidine sites, the properties of specialized DNA polymerase, and biological selection, it has been suggested that V600E could arise from error-prone replication of UV-damaged DNA.37 On the other hand, UVA absorption, being an important source of DNA oxidative damage, by generating reactive oxygen species (ROS), can greatly increase replicative errors of different types, including T/A base substitution. This oxidative damage is further implemented by inflammation processes, which is generally induced by UV sunburns.38 Although, at present, it is difficult to assess how UV is involved in the onset of BRAF mutation, repeated events of intense sun exposure have been correlated with the accumulation of V600E in nevi and melanomas. This mechanism is consistent with our results showing a quantitative association between the sun exposure and the fraction of alleles carrying BRAFV600E. A convincing example of this evidence is provided by those patients with two nevi obtained from distant, but sun-exposed sites. In these cases, both the lesions showed a similar fraction of V600E mutated cells.
In summary, our study shows that in acquired nevi and cutaneus melanomas, BRAFV600E is an early event affecting a variable percentage of nevocytic and melanocytic alleles. The entity of this fraction depends on the type and history of sun exposure and is unrelated to the patients' phototype. According to our results, the onset of BRAFV600E is independent of the progression and unrelated to the MAPK pathway activation, which is achieved only later, following the acquisition of other genetic alterations. As a consequence, in these lesions, BRAFV600E can be regarded as a genetic hallmark of DNA damage induced by sun exposure. This mutation can lead to transformation by mechanisms alternative to cell proliferation.
Accession codes
References
Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet 2003;33:19–20.
Kumar R, Angelini S, Snellman E, et al. BRAF mutations are common somatic events in melanocytic nevi. J Invest Dermatol 2004;122:342–348.
Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res 2003;9:6483–6488.
Yazdi AS, Palmedo G, Flaig MJ, et al. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol 2003;121:1160–1162.
Chang DZ, Panageas KS, Osman I, et al. Clinical significance of BRAF mutations in metastatic melanoma. J Transl Med 2004;42:46.
Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras–Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog 2004;3:6.
Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneus melanomas. Clin Cancer Res 2004;10:1753–1757.
Saldanha G, Purnell D, Fletcher A, et al. High BRAF mutation frequency does not characterize all melanocytic tumor types. Int J Cancer 2004;111:705–710.
Sasaki Y, Niu C, Makino R, et al. BRAF point mutations in primary melanoma show different prevalences by subtype. J Invest Dermatol 2004;123:177–183.
Poynter JN, Elder JT, Fullen DR, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res 2006;16:267–273.
Goel VK, Lazar AJ, Warneke CL, et al. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneus melanoma. J Invest Dermatol 2006;126:154–160.
Omholt K, Karsberg S, Platz A, et al. Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneus melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res 2002;8:3468–3474.
Smalley KS . A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer 2003;104:527–532.
Uribe P, Andrade L, Gonzalez S . Lack of association between BRAF mutation and MAPK ERK activation in melanocytic nevi. J Invest Dermatol 2006;126:161–166.
Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005;436:720–724.
Merlino G, Noonan FP . Modeling gene-environment interactions in malignant melanoma. Trends Mol Med 2003;9:102–108.
Jhappan C, Noonan FP, Merlino G . Ultraviolet radiation and cutaneus malignant melanoma. Oncogene 2003;22:3099–3112.
Pfeifer GP, You YH, Besaratinia A . Mutations induced by ultraviolet light. Mutat Res 2005;571:19–31.
Jiveskog S, Ragnarsson-Olding B, Platz A, et al. N-ras mutations are common in melanomas from sun-exposed skin of humans but rare in mucosal membranes or unexposed skin. J Invest Dermatol 1998;111:757–761.
Cohen Y, Rosenbaum E, Begum S, et al. Exon 15 BRAF mutations are uncommon in melanomas arising in nonsun-exposed sites. Clin Cancer Res 2004;10:3444–3447.
Edwards RH, Ward MR, Wu H, et al. Absence of BRAF mutations in UV-protected mucosal melanomas. J Med Genet 2004;41:270–272.
Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst 2003;95:1878–1890.
Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–2147.
Fitzpatrick TB . The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124:869–871.
Edlundh-Rose E, Egyhazi S, Omholt K, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res 2006;16:471–478.
Ichii-Nakato N, Takata M, Takayanagi S, et al. High frequency of BRAFV600E mutation in acquired nevi and small congenital nevi, but low frequency of mutation in medium-sized congenital nevi. J Invest Dermatol 2006;126:2111–2118.
Hes FJ, Nielsen M, Bik EC, et al. Somatic APC mosaicism: an underestimated cause of polyposis coli. Gut 2008;57:71–76.
Spittle C, Ward MR, Nathanson KL, et al. Application of a BRAF pyrosequencing assay for mutation detection and copy number analysis in malignant melanoma. J Mol Diagn 2007;9:464–471.
Tanami H, Imoto I, Hirasawa A, et al. Involvement of overexpressed wild-type BRAF in the growth of malignant melanoma cell lines. Oncogene 2004;23:8796–8804.
Small GW, Shi YY, Edmund NA, et al. Evidence that mitogen-activated protein kinase phosphatase-1 induction by proteasome inhibitors plays an antiapoptotic role. Mol Pharmacol 2004;66:1478–1490.
Trakul N, Menard RE, Schade GR, et al. Raf kinase inhibitory protein regulates Raf-1 but not B-Raf kinase activation. J Biol Chem 2005;280:24931–24940.
Udart M, Utikal J, Krahn GM, et al. Chromosome 7 aneusomy. A marker for metastatic melanoma? Expression of the epidermal growth factor receptor gene and chromosome 7 aneusomy in nevi, primary malignant melanomas and metastases. Neoplasia 2001;3:245–254.
Huntington JT, Shields JM, Der CJ, et al. Overexpression of collagenase 1 (MMP-1) is mediated by the ERK pathway in invasive melanoma cells: role of BRAF mutation and fibroblast growth factor signaling. J Biol Chem 2004;279:33168–33176.
Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev 2007;16:991–997.
Landi MT, Bauer J, Pfeiffer RM, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science 2006;313:521–522.
Tadokoro T, Kobayashi N, Zmudzka BZ, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J 2003;17:1177–1179.
Thomas NE . BRAF somatic mutations in malignant melanoma and melanocytic naevi. Melanoma Res 2006;16:97–103.
Ohshima H, Tazawa H, Sylla BS, et al. Prevention of human cancer by modulation of chronic inflammatory processes. Mutat Res 2005;591:110–122.
Acknowledgements
We thank Dr Giovanni Ponti for the helpful discussions. We also thank Mrs Katia Pollato for her technical assistance. This study was supported by a research grant from Regione Piemonte, for the project ‘Melanoma’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
The authors state no conflict of interest.
Rights and permissions
About this article
Cite this article
Venesio, T., Chiorino, G., Balsamo, A. et al. In melanocytic lesions the fraction of BRAFV600E alleles is associated with sun exposure but unrelated to ERK phosphorylation. Mod Pathol 21, 716–726 (2008). https://doi.org/10.1038/modpathol.2008.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.41
Keywords
This article is cited by
-
Association between BRAF V600E and NRAS Q61R mutations and clinicopathologic characteristics, risk factors and clinical outcome of primary invasive cutaneous melanoma
Cancer Causes & Control (2014)
-
Oxidative DNA damage drives carcinogenesis in MUTYH-associated-polyposis by specific mutations of mitochondrial and MAPK genes
Modern Pathology (2013)
-
Driver Mutations in Melanoma: Lessons Learned From Bench-to-Bedside Studies
Current Oncology Reports (2012)
-
Stability of BRAF V600E mutation in metastatic melanoma: new insights for therapeutic success?
British Journal of Cancer (2011)