Abstract
Acute graft-versus-host disease (aGVHD) remains a major complication following allogeneic hematopoietic cell transplantation, limiting the success of this therapy. We previously reported that interleukin-22 (IL-22) participates to aGVHD development, but the underlying mechanisms of its contribution remain poorly understood. In this study, we analyzed the mechanism of the pathological function of IL-22 in intestinal aGVHD. Ex-vivo colon culture experiments indicated that IL-22 was able to induce Th1-like inflammation via signal transducer and activator of transcription factor-1 (STAT1) and CXCL10 induction in the presence of type I interferon (IFN). To evaluate a potential synergy between IL-22 and type I IFN in aGVHD, we transplanted recipient mice, either wild-type (WT) or type I IFN receptor deficient (IFNAR−/−), with bone marrow cells and WT or IL-22 deficient (IL-22−/−) T cells. We observed a decreased GVHD severity in IFNAR−/− recipient of IL-22−/− T cells, which was associated with a lower level of STAT1 activation and reduced CXCL10 expression in the large intestine. Finally, immunohistochemistry staining of STAT1 performed on gastrointestinal biopsies of 20 transplanted patients showed exacerbated STAT1 activation in gastrointestinal tissues of patients with aGVHD as compared with those without aGVHD. Thus, interfering with both IL-22 and type I IFN signaling may provide a novel approach to limit aGVHD.
Similar content being viewed by others
Introduction
Acute graft-versus-host disease (aGVHD) remains a major complication following allogeneic hematopoietic cell transplantation (allo-HCT), limiting the success of this therapy.1 Many pro-inflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor (TNF-α), IL-12, and interferon-γ (IFN-γ) secreted following the conditioning regimen have been linked to aGVHD initiation and pathophysiology.2, 3 IL-22 is a cytokine produced by adaptive immune cells such as CD4+ (Th1, Th17, and Th22) and CD8+ (Tc17 and Tc22) T cells, and by innate immune cells such as γδ T cells or innate lymphoid cells as well.4 The IL-22 receptor is essentially expressed by nonhematopoietic cells and more particularly by epithelial cells of the gastrointestinal tract, keratinocytes, and epithelial cells of the respiratory tract.5 We recently reported that donor-derived IL-22 contributes to the severity of aGVHD in an experimental mouse model of allo-HCT using IL-22-deficient mice (IL−/−).6 IL-22 is a member of the IL-10 family that exerts both protective and inflammatory functions, most likely depending on the cytokine microenvironment and the tissues and/or the cell types involved.7 The binding of IL-22 on its receptor activates signal transducer and activator of transcription factor 3 (STAT3) signaling and induces expression of antimicrobial peptides, as well as cell proliferation and tissue repair.8 However, during intestinal inflammation, when adaptive immune responses are overwhelming, IL-22 secreted by T cells can be pathogenic by causing mucosal hyperplasia.9 Exuberant IL-22 production together with that of other proinflammatory cytokines might create a cytokine milieu that promotes tissue inflammation. Consistent with this notion, a recent study by Bachmann et al.10 reported a synergy between IFN-α and IL-22, which leads to the activation of STAT1 in human colonic cell lines. Such synergy is likely to contribute to inflammatory mechanisms in several diseases involving both cytokines.11 This ambivalent role of IL-22 depending on cytokine environment is in line with data showing that IL-22 was involved in gut lesions after Toxoplasma gondii infection and development of Th1 cytokine-mediated inflammation.12 The inflammatory milieu resulting from the allo-HCT procedure and, in particular, the conditioning regimen leading to the induction of the so-called “cytokine storm” may favor the pathological function of IL-22 rather than its tissue-protective effect. Moreover, IL-22 activity is regulated by IL-22-binding protein, a soluble IL-22 receptor that prevents IL-22 binding to its receptor.13 In this report, we determined the factors involved in the pathological effect of IL-22 in the setting of acute intestinal GVHD.
Results
Graft-derived IL-22 increases STAT1 phosphorylation and inflammatory chemokine production in the colon of recipient mice during aGVHD
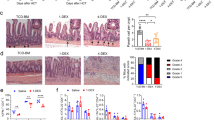
We previously demonstrated that donor-derived IL-22 was associated with aGVHD severity in a full major histocomatibility complex-mismatched GVHD model.6 This was attested by a decrease of GVHD lesions in the small and large intestine, as well as reduced pro-inflammatory factor expression in recipient mice receiving IL-22−/− donor T cells. These data are notably in line with a recent report confirming the pathogenic role of IL-22 in murine GVHD.14 Here we assessed the role of the engagement of pathological STAT1 pathway in IL-22-induced GVHD. Thus, administration of B6 IL-22−/− T cells with T-cell-depleted bone marrow (BM)into lethally irradiated allogeneic BALB/c recipient mice significantly decreased GVHD mortality and GVHD scores as compared with recipients receiving wild-type (WT) allogeneic T cells (Figure 1a,b). Next, mRNA transcripts of inflammatory mediators such as CXCL9 and CXCL10 were also analyzed in colonic tissue, with a significant reduction of cxcl10 expression and a trend toward a reduction of cxcl9 expression in mice that received IL-22−/− T cells (Figure 1c,d). As CXCL9 and CXCL10 are known to be induced by STAT1 activation, we measured STAT1 phosphorylation in the colon of transplanted mice. Western blot analyses of proteins extracted from colon tissue isolated at day 7 post-bone-marrow-transplantation (BMT) revealed that phosphorylated STAT1 (P-STAT1) was induced in samples from mice that received allogeneic WT T cells, as compared with mice receiving IL-22−/− T cells or BM control (Figure 1e). This result was also confirmed by cytometric bead array kit analysis, showing that the P-STAT1/t-STAT1 ratio was significantly higher in the colon from mice administrated with WT T cells, as compared with those receiving IL-22−/− T cells (Figure 1f) or BM cells alone. Altogether, these results suggest that the STAT1 pathway is involved in acute intestinal GVHD and donor-derived IL-22 contributes to STAT1 activation signaling.
Graft-derived interleukin-22 (IL-22) increases signal transducer and activator of transcription factor-1 (STAT1) phosphorylation and inflammatory cytokine production in the colon of recipient mice during acute graft-versus-host disease (aGVHD). Lethally irradiated BALB/c recipients were transplanted with 5 × 106 B6.WT T cell-depleted bone marrow (TCD BM) only or with 1 × 106 B6.WT T cells or 1 × 106 B6.IL-22−/− T cells (n=5 per group and three independent experiments). (a) Survival data are shown (log-rank test, P<0.05 for BM+WT T cells vs. BM+ IL-22 deficient (IL-22−/−) T cells). (b) GVHD clinical scores were determined as described in Methods and were significantly decreased in BM+IL-22−/− T cell recipients at day 9 post allogeneic hematopoietic cell transplantation (allo-HCT), P<0.005 (c,d). Quantitative reverse transcriptase–PCR analysis for each indicated gene was performed using RNA isolated from the large intestine at day 7 after allo-HCT. Mean±s.e.m. of gene expression levels are represented. Data are combined from at least three independent experiments (n=12). (e,f) Total protein was isolated from the large intestine collected at day 7 after allo-HCT. (e) Cellular content of phosphorylated (P) STAT1, total (t) STAT1, and β-actin was evaluated by western blot analysis. One representative experiment is shown. (f) P-STAT1/t-STAT1 ratio was evaluated using the Cell Signaling cytometric bead array kit (CBA) (n=3 per group and 6 independent experiments).
IL-22 induces STAT1 phosphorylation and inflammatory cytokine in the presence of IFN-α in the colon
IL-22 has been shown to trigger different types of responses depending on the cytokine environment.15 Moreover, recent data demonstrated the effects of type I IFN priming on IL-22-activated STAT1 in human colon cell lines.10 To evaluate this effect on IL-22-mediated STAT1 activation, we used an ex-vivo colon culture model as previously described.16 Colon section samples were isolated from naive B6 IL-22−/− mice in order to limit a potential effect of endogenous IL-22. The sections were cultured for 24 h in the presence of recombinant cytokines IFN-α, IL-22, or both. We measured the expression of RegIIIγ, an antimicrobial peptide produced in response to IL-22, as positive control for IL-22 stimulation. In IL-22−/− colon, RegIIIγ expression was not detectable in the absence of IL-22, whereas a strong induction was observed after recombinant IL-22 treatment (Figure 2a). As a positive control for recombinant IFN-α response, irf7 expression was assessed after recombinant IFN-α stimulation, as previously described17 (Figure 2b). To examine the synergistic effect of type I IFN and IL-22 on the large intestine, we next analyzed the expression of the chemokines CXCL9, CXCL10, and CXCL11. We observed a significant induction of cxcl10 mRNA after IFN-α and IL-22 treatment, as compared with IFN-α or IL-22 alone (Figure 2c). We also observed a trend toward an increase of cxcl11 after both IL-22 and IFN-α stimulation (Figure 2d), whereas no difference was observed for cxcl9 (Figure 2e) or TNF-α expression (Figure 2f). Moreover, the analysis of STAT1 activation by western blotting revealed that both P-STAT1 and t-STAT1 were induced after IL-22 and IFN-α stimulation (Figure 2g), which is in line with previous data on colon cell lines.10 Finally, the use of ruxolitinib, an inhibitor of JAK1/2 or tofacitinib, an inhibitor of JAK1/3 located upstream of the STAT1 signaling pathway,18 completely abolished STAT1 activation (Figure 2h) and cxcl10 mRNA induction mediated by both IFN-α and IL-22 (Figure 2i) in colon cultures. These results show that IL-22 can increase cxcl10 mRNA expression and activate STAT1 in the large intestine in the presence of type I IFN. To evaluate whether the cytokines IL-22 and IFN-α were able to cooperate on the same cells, we performed experiments using intestinal cell lines stimulated with IL-22 or IFN-α alone, or both. We analyzed the activation of STAT1 and STAT3 by flow cytometry at different time points after stimulation. As shown in Figure 3a, we observed that the activation of STAT1 peaked at 15 min after addition of IFN-α, then decreased at later time points. We also observed that IL-22 induced the activation of STAT1 to a lesser degree than IFN-α, as previously described (Figure 3b), whereas only IL-22 was able to induce STAT3 activation (Figure 3c). We observed that STAT1 activation was significantly higher at 15 min after addition of both IL-22 and IFN-α, demonstrating the synergy between these two cytokines on epithelial cells. In addition, when IL-22 was added at 15 h after priming with IFN-α, when STAT-1 activation returned to the basal level, IL-22 induced a strong activation of STAT1 (Figure 3d) as well as an increased production of the chemokine CXCL10 (Figure 3f). These results suggest that IL-22 can amplify but also sustain the IFN-α-induced STAT1 activation and CXCL10 expression.
Combination of interleukin-22 (IL-22) and interferon-α (IFNα) increases signal transducer and activator of transcription factor-1 (STAT1) phosphorylation and inflammatory cytokine production in the large intestine. Colons from IL-22 deficient (IL-22−/−) B6 naive mice were excised, prepared and cultured as described in Methods. IL-22−/− colon segments were either kept as unstimulated control or stimulated with IL-22 (100 ng ml−1) for 18 h (a–f) or 45 min (g). Where indicated, colon segments were preincubated with IFN-α (5 × 104 U ml−1) for 12 h. (a–f) Reg3γ, IRF7, CXCL9, CXCL10, CXCL11, and TFN-α mRNA expression were assessed by real-time PCR analysis. Relative expressions were normalized to glyceraldehydes 3-phosphate dehydrogenase (GAPDH; n=3 per group and 3 independent experiments). *P<0.05, **P<0.01. (g) Cellular content of phosphorylated (P) STAT1, total (t) STAT1 and β-actin was evaluated by western blot analysis. One representative of at least three independent experiments is shown. (h,i) Colon segments were preincubated for 12 h with IFN-α (5 × 104 U ml−1) alone or, where indicated, the JAK inhibitors ruxolitinib (RUXO, 10 μM) or tofacitinib (TOFA, 10 μM), or control vehicle (dimethyl sulfoxide (DMSO)). After preincubation, colon segments were either kept as unstimulated control or stimulated with IL-22 (100 ng ml−1) for 45 min (h) or 18 h (i). (h) Cellular content of phosphorylated (P) STAT1, total (t) STAT1, and β-actin was evaluated by western blot analysis. One representative of at least two independent experiments is shown. (i) CXCL10 mRNA expression was assessed by real-time PCR analysis. Relative expression was normalized to GAPDH (n=3 per group and 3 independent experiments).
Interleukin-22 (IL-22)/type I interferon (IFN) synergy increases signal transducer and activator of transcription factor (STAT)1 but not STAT3 activation in intestinal epithelial cells (IEC). (a,b) Intestinal epithelial HT29 cells were cultured in the presence of both IL-22 and IFN-α added at the same time. Phosphorylated STAT (P-STAT) analyses were performed by flow cytometry. (a) Data represent cells positive for the indicated P-STAT molecule after 15 min of culture in the presence of IL-22 alone (green), IFN-α alone (blue) or IL-22 and IFN-α (red) as compared as non-stimulated condition (grey). Data represent one of three independent experiments. (b) Data represent mean±s.e.m. of the ratio of the mean of fluorescence intensity (MFI) of stimulated cells with the unstimulated condition at different time points (n=3). (c–e) Where indicated, intestinal epithelial HT29 cells were cultured for 15 h in the presence of IFN-α. (c,d) Where indicated, IL-22 was added to the medium for 30 min before P-STAT staining. (e) Where indicated, IL-22 was added to the medium for 24 h and culture supernatant hCXCL10 analyses were performed by enzyme-linked immunosorbent assay. *P<0.05, ***P<0.001.
The absence of IL-22 in donor cells and type I IFN signaling pathway in recipient mice decrease GVHD severity in a minor histocompatibility antigen-mismatched model
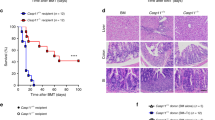
We next addressed how engagement of STAT1 activation induced by both IL-22 and type I IFN can affect GVHD severity. For this purpose, we transferred BM and splenocytes from WT or IL-22−/− B6 mice into lethally irradiated WT or type I IFN receptor deficient (IFNAR−/−) 129 mice (Figure 4a). In this minor histocompatibility antigen-mismatched model, recipient mice developed GVHD starting around day 14 post transplantation. In order to better characterize this GVHD model, we evaluated intestinal barrier integrity in BMT recipients by performing oral administration of fluorescein isothiocyanate (FITC)–dextran, at day 8 and day 20 after BMT. We observed an increase of GVHD-related intestinal damage and loss of epithelial barrier integrity in mice receiving spleen cells as compared with BM control, indicating that in the B6 to 129 model, aGVHD is associated with intestinal lesions (Figure 4b,c). This loss of epithelial barrier integrity was confirmed with an increase of pathological scores in the colon at day 20 between the control group and GVHD groups (Figure 4d,e). As expected, transplantation with IL-22−/− cells was associated with a less severe form of GVHD with a decrease of histological score (Figure 4d,e) and weight loss (Figure 4f). Interestingly, in this minor histocompatibility antigen-mismatched GVHD model, only the absence of IL-22 in donor T cells combined with IFNAR deficiency in recipients led to a statistically significant reduction of intestinal barrier destruction as shown by the reduction of FITC–dextran (Figure 4c) and of GVHD-induced mortality, as compared with that of WT mice receiving WT splenocytes (median survival time undefined versus 26 days, P<0.05) (Figure 4g).
The absence of interleukin-22 (IL-22) in donor cells and type I IFN receptor (IFNAR) in recipient mice decreases graft-versus-host disease (GVHD) severity in a minor histocompatibility antigen-mismatched GVHD model. (a–g) Lethally irradiated wild type (WT) or IFNAR deficient (IFNAR−/−) 129 recipients were transplanted with 5 × 106 B6 WT or IL-22 deficient (IL-22−/−) bone marrow (BM) only or with 10 × 106 B6.WT splenocytes or 10 × 106 B6.IL-22−/− splenocytes (n=5 per group and five independent experiments). (b,c) WT or IFNAR−/− 129sv mice transplanted with BM only, WT graft or IL-22−/− graft were challenged with oral gavage of fluorescein isothiocyanate (FITC)–dextran at day 8 (b) and 20 after allogeneic hematopoietic cell transplantation (allo-HCT) (c). Graph shows plasma FITC–dextran concentrations. Data are combined from at least four independent experiments (n=12). (d,e) Pathology scores in the colon of recipient mice 20 days after allo-HCT. (d) Data are presented as the mean±s.e.m. from cumulative results from four experiments. (e) A representative example of the section of the large intestine of 129sv mice, WT or IFNAR−/−, transplanted with BM only or B6.WT cells or B6.IL-22−/− cells at day 20 ( × 200 magnification). (f) Mean±s.e.m. of percentage of initial weight measured at day 20 after allo-HCT are represented. (g) Survival data are shown (log-rank test, P<0.05 for B6.WT graft in WT 129sv recipients versus B6.IL-22−/− graft in IFNAR−/− 129sv). Data are combined from at least five independent experiments (n=20).
Altogether, these results suggest that donor-derived IL-22 and type I IFN signaling may be critical for IL-22 pathogenicity in the intestinal aGVHD process.
The absence of IL-22 in donor cells and type I IFN signaling pathway in recipient mice lead to reduction of inflammatory cytokines
The data presented above showed that the absence of donor-derived IL-22 and of type I IFN signaling pathway in recipient organs lowered GVHD severity. The reduced mortality of recipient mice was accompanied by a reduced expression of circulating pro-inflammatory mediators, as detected in the serum of transplanted mice at day 8 after BMT (Figure 5a–c). Significantly reduced systemic levels of Th1-associated cytokines (TNF-α and IFN-γ) were observed only in IFNAR−/− mice receiving IL-22−/− T cells, but not of IL-6. These observations confirm the combinatory role of donor IL-22 and type I IFN signaling pathway in GVHD triggering.
Interleukin-22 (IL-22) in donor cells and intact type I interferon (IFN) signaling pathway in recipient mice are required for increased inflammatory cytokines production. (a–c) Plasma was collected at day 20 after allogeneic hematopoietic cell transplantation (allo-HCT) and cytokine concentrations were measured using a cytometric bead array kit (CBA) set. Data from at least three independent experiments with three mice per group are shown. Mean is indicated by a bar.
The absence of IL-22 in donor cells and type I IFN signaling pathway in recipient mice lead to reduction of Th1 inflammation in recipient intestine
As IL-22 and type I IFN can increase Th1-like inflammation in colon explants via CXCL10, we first evaluated the expression of CXC-chemokine receptor 3 (CXCR3), the receptor for CXCL10 on T cells from mesenteric lymph nodes of mice experiencing GVHD. As shown in Figure 6, we observed an increase in the proportion of T cells expressing CXCR3 in mice, indicating that these T cells can respond to CXCL10 and migrate to intestinal tissues according to the level of CXCL10 produced in situ (Figure 6a,b). Therefore, we evaluated cxcl10 expression in recipient intestine in GVHD context. Interestingly, the expression of cxcl10 and the Th1-associated cytokine ifn-γ were significantly decreased in the colon of IFNAR−/− recipients in the absence of donor IL-22 (Figure 6c,d). The most significant decrease of CXCL10 and ifn-γ expression was observed in the group of IFNAR−/− mice transplanted with IL-22−/− graft, as compared with WT graft (P<0.001) (Figure 6c,d). No significant differences in CXCL10 expression was observed in WT recipient transferred with IL-22−/− graft or in IFNAR−/− recipient transferred with WT graft as compared with the WT group (Figure 6c).
Expression of CXC-chemokine receptor 3 (CXCR3) is increased in Th cells of mice experiencing intestinal graft-versus-host disease (GVHD). (a–d) Lethally irradiated wild-typr (WT) or type I IFN receptor deficient (IFNAR−/−) 129sv recipients were transplanted with 5 × 106 B6 WT or IL-22 deficient (IL-22−/−) bone marrow (BM) only or with 10 × 106 B6.WT splenocytes or 10 × 106 B6.IL-22−/− splenocytes (n=5 per group and five independent experiments). At day 20 post transplantation, mesenteric lymph nodes (MLNs) were collected. Extracellular expression of CXCR3 was analyzed CD4+ T cells by flow cytometry. (a) Expression of CXCR3 on gated CD4+ T cells is shown for a representative sample. (b) Mean±s.e.m. of the percentage of CD4+ T cells expressing CXCR3 in the MLNs is shown. (c,d) At day 20 post transplantation, colons were excised for quantitative reverse transcriptase–PCR analysis for each indicated gene. Data are combined from three independent experiments (n=12).
We also evaluated the profile of cytokines produced by allogeneic T cells in the mesenteric lymph nodes draining the intestine of mice at day 20 after transplantation. We observed a trend to a reduction of IL-17A+IFN-γ T cells among CD4+ T cells from the mesenteric lymph node (Figure 7a and Supplementary Figure S1 online), but these differences were not significant (mean±s.e.m.: WT→WT: 5.83±0.9; IL-22−/−→WT: 6.35±1; WT→IFNAR−/−: 2.77±0.5; IL-22−/−→IFNAR−/−: 2.66±0.32; P=0.23, WT→WT and IL-22−/−→IFNAR−/−), whereas a significant decrease of IFN-γ+ CD4+ T cells was observed in the group of IFNAR−/− mice transplanted with IL-22−/− graft, as compared with the WT graft. Such difference was not observed for WT mice receiving IL-22−/− T cells (Figure 7b). In addition, we also observed a similar significant decrease of IFN-γ+ CD4+ T cells in the spleen (Figure 7c). Thus, in vivo T-cell proliferation was assessed by Ki67 staining of T cells from the spleen at day 20 after transplantation. Despite the increase in Ki67+ CD4+ T cells and, more importantly, Ki67+ CD8+ T cells in mice developing GVHD compared with BM control mice, no difference was observed in CD4+ or CD8+ T-cell proliferation between WT and IFNAR−/− recipient (Supplementary Figure S2). This suggests that in this model, type I IFN does not favor donor CD4+ or CD8+ T-cell proliferation. In addition, we did not observe any difference in the proportion of CD25+Foxp3+ regulatory T cell among CD4+ T cells from the spleen of WT mice that received WT spleen cells, as compared with IFNAR−/− mice that received IL-22−/− splenocytes (data not shown). Overall, this suggests that the effect of both graft-derived IL-22 and type I IFN deficiency in recipient mice affects mainly GVHD target tissues, rather than directly T cells. This is in line with the restricted expression of IL-22R1 on these target tissues.
Interleukin-22 (IL-22) in donor cells and intact type I interferon (IFN) signaling pathway in recipient mice are required for increased inflammatory mediator production. (a–c) Lethally irradiated wild-type (WT) or type I IFN receptor deficient (IFNAR−/−) 129sv recipients were transplanted with 5 × 106 B6 WT or IL-22-deficient (IL-22−/−) BM only or with 10 × 106 B6.WT splenocytes or 10 × 106 B6.IL-22−/− splenocytes (n=5 per group). Spleen and mesenteric lymph nodes (MLNs) were collected at day 20 post allogeneic hematopoietic cell transplantation (allo-HCT). Splenocytes and MLN cells were activated for 6 h with phorbol-12-myristate-13-acetate (PMA), ionomycin, and brefeldin A. Intracellular IFN-γ and IL-17 in CD4+ T cells were analyzed. (a) Expression of IL-17 and IFN-γ on gated CD4+ T cells is shown for a representative sample. (b,c) Mean±s.e.m. of the percentage of CD4+ T cells secreting IFN-γ (IFN-γ+IL-17+ and IFN-γ+IL-17−) in the MLN and spleen is shown. Data are combined from three independent experiments (n=15).
P-STAT1 and CXCL10 are increased in human intestinal GVHD lesions
Next, we evaluated the activation of STAT1 signaling pathway on intestinal biopsies from a group of 20 transplanted patients with and without GVHD. As shown by immunohistochemical staining, enhanced P-STAT1 was observed in biopsies from patients developing aGVHD as compared with transplanted patients without aGVHD (Figure 8a–d). Furthermore, in aGVHD biopsies P-STAT1 was expressed by epithelial cells and in chorionic mononuclear cells (Figure 8c). Interestingly, we also observed an increase in CXCL10 staining in biopsies from patients with gastrointestinal GVHD (Figure 8e–g). Thus, these observations suggest that STAT1 and CXCL10 signaling pathway is also critical in human intestinal GVHD.
Signal transducer and activator of transcription factor-1 (STAT1) activation and CXCL10 expression are increased during gastrointestinal (GI) graft-versus-host disease (GVHD) in patients in both intestinal epithelial cells and mononuclear cells of the lamina propria around altered crypts. (a–g) STAT1 phosphorylation and CXCL10 expression are observed in cells from patients with severe GI acute GVHD (aGVHD), but not control patients. (a–c) Representative immunohistochemistry of phosphorylated STAT1 (P-STAT1) in the GI tissue (original magnification, × 200 for a and b, and × 400 for c). (d) Expression of P-STAT1 is represented. (e,g) Representative immunohistochemistry of CXCL10 in GI tissue (original magnification × 200 for e and f). (g) Expression of CXCL10 is represented.
Discussion
In this study, we confirmed IL-22 as a critical component for the development of acute intestinal GVHD in both major and minor histocompatibility-mismatched models. In fact, both protective and inflammatory properties have been described for IL-22, depending on the cytokine microenvironment and the tissues and/or cell types. Thus, it has been reported that sustained IL-22 systemic exposure leads to an acute-phase response.19 Moreover, IL-22 produced by innate lymphoid cell in the intestinal tract has been observed as a protective factor in several diseases. Nevertheless, in the aGVHD context, Hanash et al.20 showed that innate lymphoid cell are completely depleted following donor CD4+ T-cell infiltration in the lamina propria, which is consistent with a recent report showing that conventional CD4+ T cells reduce innate lymphoid cell-3 number in lamina propria in a microbiota and inflammation-independent manner.21 Other studies have described that IL-22 can be pathogenic, notably in synergy with other cytokines such as, IL-17A15, 22 or type I IFN.10 Consistent with it, we observed a synergy between IL-22 and IFN-α in an ex-vivo mouse colon explant treatment, leading to an increase in STAT1/CXCL10 signaling pathway. Our results obtained with stimulation of intestinal epithelial cells suggest that the activation of the two signaling pathways occurs in a dependent manner on the same cells. The mechanisms underlying such IL-22/type I IFN synergy could be related to the direct interaction between their relative receptors. Indeed, it has previously been demonstrated that IFNAR can physically interact with IFN-γ receptor and facilitate PSTAT1 homodimer generation. A similar interaction has also been reported between IFNAR and IL-6 receptor (gp130).23, 24 It should be interesting to determine whether such interaction is physiologically relevant between IFNAR and IL-22 receptor.
Altogether, this suggests that IL-22 can promote Th1 cell infiltration—the main pathological T-cell subset in aGVHD—in local tissues in an IFN-α-rich environment via STAT1 and CXCL10.
Our results clearly established that graft-derived IL-22 acts in synergy with type I IFN signaling in target tissues and influence GVHD severity via STAT1 activation. These observations are consistent with previous work showing that induction of GVHD leads to a rapid phosphorylation of STAT1 in both the liver and spleen in a mouse model.25 Moreover, that study showed that treatment with histone deacetylase resulted in a significantly reduced GVHD associated with systemic and local inhibition of P-STAT1 and attenuated proinflammatory cytokine production during the initiation phase of GVHD.25 In addition, STAT1 activation by type I IFN has been reported to be a critical step in oral mucosa inflammation in chronic GVHD patients. In fact, patients with severe chronic GVHD showed elevated P-STAT1 levels in keratinocytes compared with patients without oral lesions.26 In this context, STAT1 activation was associated with an increase of inflammatory mediators such as CXCL9 and type I IFN-driven migration, proliferation, and differentiation of T-cell effectors.26 Our observation of STAT1 activation in GVHD patients are in line with previous results showing that the expression and the activation of STAT1 was heightened in colonic mucosa of patients with ulcerative colitis.27 A recent report by Capitini et al.28 demonstrated that transplantation of STAT1-deficient BM resulted in a decrease of GVHD dependent on functional alteration of plasmacytoid dendritic cells and IFN-α production. In addition, pharmacologic inhibition of STAT1 also significantly reduced GVHD severity, thus demonstrating the effectiveness of this strategy. The use of Jak1/2 inhibitors or the development of STAT1 inhibitors may constitute promising therapeutic tools for GVHD treatment as suggested by a recent report showing that ruxolitinib can decrease the proliferation of alloreactive T cells.29 Here we observed that Jak1/2 inhibitor can also limit the production of proinflammatory chemokines in host tissues, which increases the potential of these molecules in aGVHD treatment. The impact of type I IFN in murine GVHD has been reported to be pathogenic according to the experimental model.30 Although we did not demonstrate the origin of the cells producing type I IFN after BMT, we cannot exclude that plasmacytoid dendritic cells are involved in this process. Interestingly, we previously demonstrated that plasmacytoid dendritic cellsare increased in the gastrointestinal mucosa of GVHD patients.31 The recent report from Zhao et al.32 showed that the main producers of IL-22 involved in alloreactive immune responses of GVHD were donor CD4+ T cells that carried CD62L−CD44high/low surface markers, corresponding to effector memory or recent activated T cells. Accordingly, we also reported that IL-22 is produced by donor CD4+ T cells in mesenteric lymph nodes in early stage of GVHD.6
In our study, we showed that CXCL10 was increased in response to the combination of IL-22 and type I IFN effect. CXCL10 can recruit effector T cells expressing CXCR3 to sites of tissue injury. Moreover, the inhibition of CXCR3 has been shown to reduce the severity of GVHD in mice.33 Interestingly, a recent report demonstrated that in addition to recruiting CXCR3+ Th1 cells, CXCL10 is also involved in Th1 polarization, whereas CXCL11 preferentially induced regulatory T cells via a STAT3–STAT6-dependent pathway suppressing autoimmune encephalomyelitis.34 Thus, we speculate that CXCL10 can amplify the Th1 response observed early after BMT and its altered production in the colon of IFNAR−/− mice receiving IL-22−/− T cells may explain the decrease of infiltrated Th1 CD4+ T cells. The Th1 cytokines IFN-γ and TNF-α have been reported to directly contribute to the loss of intestinal barrier integrity by acting on the tight junctions.35 Thus, by contributing to the local Th1 response, the synergy between donor-derived IL-22 and host type I IFN signaling pathway may favor the loss of intestinal barrier integrity in the aGVHD process. Recently, it has been shown that IL-22 promotes accumulation of neutrophils in peripheral tissues, notably in the liver and lung, via CXCL1 production, the main neutrophil chemoattractant.36 In fact, the role of neutrophils recruited in intestinal tissues of GVHD recipients contributes to tissue damage and aggravation of intestinal GVHD.37 Whether donor-derived IL-22 and host type I IFN also participates in neutrophil recruitment during GVHD remains to be studied. We propose a global model that supports our hypothesis on the cooperation between IL-22 and IFN-α in intestinal GVHD development. Indeed, the conditioning regimen would induce some significant toxicity against the intestinal barrier and bacterial translocation leading to the release of pathogens and damage-associated molecular patterns (PAMPs) dissemination, antigen-presenting-cells (APC) infiltration, and allogeneic T-cell activation. Among APC pDC can secrete type I IFN, whereas activated allogeneic T cells will produce IL-22. Both IL-22 and type I IFN can activate the STAT1 transcription factor and induce CXLC10 secretion by intestinal epithelial cells, resulting in increased Th1 infiltration at the intestinal level and thereby perpetuating increased intestinal GVHD (Supplementary Figure S3).
Altogether, our results provide insight into the mechanisms underlying the pathogenic effect of IL-22 in combination with inflammatory cytokines.
Methods
Mice. WT 129sv (hereafter named 129) (H2b), BALB/c (H2d), or C57BL/6 (hereafter named B6) (H2b) mice were purchased from Charles River (Chatillon-sur-Chalaronne, France). IL-22−/− and IFNAR−/− mice were kindly provided by J.C. Renauld (Ludwig Institute for Cancer Research, Brussels, Belgium). The IL-22−/− deficient mice were originally generated in the 129 background and were subsequently backcrossed with B6 mice for 13 generations. We next maintained breeding colonies in our animal facility. All mice were used at 8–12 weeks of age. All protocols were performed according to the approval of the “Services Vétérinaires de la Santé et de la Protection Animale” delivered by the Ministry of Agriculture (Paris, France).
HCT procedures. Recipient mice were conditioned with total body irradiation administrated as single 750 cGy (BALB/c) or 850 cGy (129) lethal doses on day-1. BALB/c recipient mice were transplanted with 5 × 106 T-cell-depleted BM from WT B6 mice and 1 × 106 splenic T cells from either WT or IL-22−/− B6 mice. T-cell depletion was performed using the CD3 MicroBead Kit (Miltenyi Biotec, Paris, France) and purified populations of donor T cells were obtained using the Pan T Cell Isolation Kit II (Miltenyi Biotec) according to the manufacturer’s instructions. The purity of T-cell-depleted BM and T cells in all experiments exceeded 95%. To induce aGVHD in 129 recipients, mice were transplanted with 5 × 106 BM and 10 × 106 donor spleen cells from either WT or IL-22−/− B6 mice. Cells were injected in the lateral tail vein.
Assessment of aGVHD. aGVHD was assessed by a clinical scoring system and by histological analysis of the small and large intestine, as previously described.6 Intestinal barrier permeability was assessed using FITC–dextran (FD4-1G, Sigma-Aldrich, Lyon, France) administered by oral gavage at a concentration of 80 mg ml−1 in phosphate-buffered saline in 200 μl (16 mg) per mouse. Four hours later, plasma was collected from the peripheral blood, then mixed 1:1 with phosphate-buffered saline, and analyzed on a plate reader at excitation/emission wavelength of 485 nm per 535 nm.
Immunohistochemistry of human intestinal biopsies. Intestinal biopsies were collected from a cohort of 16 patients who underwent allo-HCT at the University-Hospital of Nantes (Nantes, France) between May 2007 and December 2010. Patients included in this study were diagnosed with gastrointestinal aGVHD by histological examination of biopsies. All patients were enrolled in clinical research protocols approved by the local ethical committees. The control patients were a cohort already published of five allo-HCT patients who volunteered to participate to the study and did not have digestive symptoms. Written informed consent was obtained in accordance with the Declaration of Helsinki. Patients, disease, and allo-HCT characteristics are summarized in Supplementary Table S1. Immunohistochemical analysis was performed on 5 μm formalin-fixed, paraffin-embedded sections using an indirect immunoperoxidase method. Staining for pSTAT1 (P-STAT1-Tyr701, clone 58D6 (Cell Signaling, Ozyme, Saint-Quentin en Yvelines, France) and CXCL10 (Peprotech, TEBU-BIO, Le-Perray-en-Yvelines, France Rabbit anti-human CXCL10) of the human biopsies was performed. The immunological reaction was visualized with the bond polymer refine detection system and 3,3-diaminobenzidine tetrahydrochloride as the chromogen as we previously described.31 In negative control experiments, the primary Abs were omitted. To evaluate STAT1 and CXCL10 expression, we used the following semi-quantitative grading scale: grade 0, no expression; grade 1, low expression; grade 2, medium expression; and grade 3, high expression. The grading of antigen expression was performed independently by two examiners (F.M. and C.B.).
Ex-vivo colon culture. Sections of 1 cm of colon were excised and cleared of feces, fat tissues, and Peyer’s patches. They were then longitudinally opened and mucus was removed by shaking in Hank’s balances slt solution containing 10% fetal bovine serum and 5 mM EDTA (Sigma-Aldrich). The colon sections were subsequently cultured in complete RPMI medium at 37 °C with 5% CO2. Where indicated, the sections were incubated with recombinant mouse IFN-α (5.104 U ml−1) (Miltenyi Biotec). Mouse recombinant IL-22 (100 ng ml−1) (R&D Systems, Lille, France) was added directly thereafter, without exchange of medium. Ruxolitinib and tofacitinib were purchased from Invivogen (Toulouse, France), and were used at the indicated concentrations. At indicated time points, the tissue sections were collected for protein or mRNA extractions.
Protein extraction. Tissue samples were homogenized using FastPrep-24 system (MP Biomedicals, Illkirch, France) and cell lysates were prepared using a protease inhibitor cocktail (cOmplete, EDTA-free, Roche, Boulogne-Billancourt, France). Total protein concentration was determined by microBCA Protein Assay (Pierce, Thermo Fischer Scientific, Courtaboeuf, France) according to the manufacturer’s instructions.
Western blot analysis. The following antibodies were used for western blotting: pSTAT1-Y701 (58D6 rabbit monoclonal antibody 1:1,000), STAT1 (rabbit polyclonal antibody 1:1,000), β-actin (D6A8, rabbit monoclonal antibody 1:1,000, Cell Signaling Technology, Ozyme, Saint-Quentin-en-Yvelines, France). Signals were detected with horseradish peroxidase-conjugated anti-Rabbit IgG (eB182) secondary antibody 1:25,000 (Rockland, Tebu-Bio, Le Perray en Yvelines, France) and quantified on a bioluminescence imager and BIO-1D advanced software (Wilber-Lourmat, Marne la Vallée, France).
Cytometric bead array kit measurement. Concentrations of IFN-γ, TNF-α, and IL-6 were measured using the BD cytometric bead array kit (BD Biosciences, Le Pont de Claix, France) according to the manufacturer’s instructions. P-STAT1 and total STAT1 (t-STAT1) concentrations were quantified using the BD cytometric bead array kit cell signaling kit (BD Biosciences) with an input of 300 μg of total protein extracted from intestinal tissue.
P-STAT fluorescence-activated cell sorting analysis. Human intestinal epithelial cell line HT29 was kindly provided by Dr Harry Sokol (Paris, France). HT-29 cells were cultured in complete Dulbecco’s modified Eagle’s medium at 37 °C with 5% CO2. Where indicated, the cells were incubated with human IFN-α (1.103 U ml−1; Roféron-A, Roche) and/or with recombinant IL-22 (100 ng ml−1; R&D Systems). Before staining, HT29 were incubated with phosphate-buffered saline-EDTA (10 mM) for 5 min and collected. Alexa647-P-STAT1 and PE-P-STAT3 (BD Biosciences) staining were performed according to manufacturer’s intruction.
hCXCL10 enzyme-linked immunosorbent assay. Concentration of hCXCL10 (Biolegend, Ozyme, Saint-Quentin-en-Yvelines, France) in cell culture supernatants was determined by enzyme-linked immunosorbent assay according to the manufacturers’ instructions.
Real-time PCR analysis. Tissue samples were homogenized using FastPrep-24 system with RLT buffer (Qiagen, Cergy-Pontoise, France) supplemented with β-mercaptoethanol (Sigma-Aldrich). RNA was extracted using RNeasy Mini kit (Qiagen). Total RNA was subjected to reverse transcription (High Capacity RNA-to-cDNA Master Mix, Applied Biosystems, Courtaboeuf, France) and quantified by real-time quantitative PCR using commercially available primer/probe sets (Assay On Demand, Applied Biosystems) and the iCycler CFX96 real-time PCR system (Life Science, Bio-Rad, Marnes-la-Coquette, France). Relative expression for the mRNA transcripts was calculated using the 1/2ΔΔCt method and GAPDH mRNA transcript as reference.
Flow cytometry analysis. At designated time points, animals were killed and organs were collected. Single-cell suspensions of the spleen and mesenteric lymph nodes were stimulated for 6 h with 25 ng ml−1 phorbol-12-myristate-13-acetate and 1 μg ml−1 ionomycin (Sigma-Aldrich), with 1 μl ml−1 Golgi Plug (BD Bioscience) before staining for flow cytometric analysis. Cells were first stained with eFluor 506 Fixable Viability Dye (eBiosciences, Paris, France) according to the manufacturer’s instructions. Surface staining was performed using FITC-conjugated CD45, PercPCy5.5-CD4 (from Biolegend), and APC-eFluor780-TCRβ antibodies (eBiosciences); cells were then fixed and permeabilized using CytoFix/CytoPerm Buffer (BD Biosciences) before staining with PE/Cy7-IFN-γ- (Biolegend), PE-IL-22-, and APC-IL-17A- (eBioscience, Paris, France)-specific antibodies or the corresponding isotype control. Ki67 and regulatory T cell cell analysis was performed by cell surface staining with FITC-CD8, V450-CD4, PeCy7-CD25, APC-CD3 (BD Biosciences), and eFluor780-TCRβ. Cells were then fixed and permeabilized with Foxp3 Staining Buffer set (eBioscience) and stained with PE-Foxp3 (eBioscience) and PercPCy5.5-Ki67 (BD Biosciences). Analysis of CXCR3 was performed by cell surface staining with BV421-CXCR3 (BD Biosciences). Analysis was performed using FACSCanto II (BD Biosciences) and Kaluza software (Beckman Coulter, Villepinte, France).
Statistical analysis. Group comparisons of data on cell populations, gene expression, cytokine levels, and pathology scores were performed using the non-parametric Mann–Whitney test. Survival curves were compared using the log-rank test. A value of P<0.05 was considered statistically significant in all experiments. Data were computed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
References
Wingard, J.R. et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 29, 2230–2239 (2011).
Antin, J.H. & Ferrara, J.L. Cytokine dysregulation and acute graft-versus-host disease. Blood 80, 2964–2968 (1992).
Mohty, M. et al. Inflammatory cytokines and acute graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Blood 106, 4407–4411 (2005).
Rutz, S., Eidenschenk, C. & Ouyang, W. IL-22, not simply a Th17 cytokine. Immunol. Rev. 252, 116–132 (2013).
Wolk, K., Kunz, S., Witte, E., Friedrich, M., Asadullah, K. & Sabat, R. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Couturier, M. et al. IL-22 deficiency in donor T cells attenuates murine acute graft-versus-host disease mortality while sparing the graft-versus-leukemia effect. Leukemia 27, 1527–1537 (2013).
Zenewicz, L.A. & Flavell, R.A. Recent advances in IL-22 biology. Int. Immunol. 23, 159–163 (2011).
Sonnenberg, G.F., Fouser, L.A. & Artis, D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390 (2011).
Kamanaka, M. et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J. Exp. Med. 208, 1027–1040 (2011).
Bachmann, M., Ulziibat, S., Hardle, L., Pfeilschifter, J. & Muhl, H. IFNalpha converts IL-22 into a cytokine efficiently activating STAT1 and its downstream targets. Biochem. Pharmacol. 85, 396–403 (2013).
Muhl, H. Pro-Inflammatory Signaling by IL-10 and IL-22: Bad Habit Stirred Up by Interferons? Front. Immunol. 4, 18 (2013).
Munoz, M. et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 206, 3047–3059 (2009).
Dumoutier, L., Lejeune, D., Colau, D. & Renauld, J.C. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 166, 7090–7095 (2001).
Zhao, K. et al. Interleukin-22 aggravates murine acute graft-versus-host disease by expanding effector T cell and reducing regulatory T cell. J. Interferon Cytokine Res. 34, 707–715 (2014).
Sonnenberg, G.F., Nair, M.G., Kirn, T.J., Zaph, C., Fouser, L.A. & Artis, D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17 A. J. Exp. Med. 207, 1293–1305 (2010).
Zenewicz, L.A., Yancopoulos, G.D., Valenzuela, D.M., Murphy, A.J., Stevens, S. & Flavell, R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957 (2008).
Izaguirre, A. et al. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74, 1125–1138 (2003).
Ihle, J.N. & Kerr, I.M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 11, 69–74 (1995).
Liang, S.C. et al. IL-22 induces an acute-phase response. J. Immunol. 185, 5531–5538 (2010).
Hanash, A.M. et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350 (2012).
Korn, L.L. et al. Conventional CD4+ T cells regulate IL-22-producing intestinal innate lymphoid cells. Mucosal. Immunol. 7, 1045–1057 (2014).
Ma, H.L. et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 118, 597–607 (2008).
Takaoka, A. et al. Cross talk between interferon-gamma and -alpha/beta signaling components in caveolar membrane domains. Science 288, 2357–2360 (2000).
Mitani, Y. et al. Cross talk of the interferon-alpha/beta signalling complex with gp130 for effective interleukin-6 signalling. Genes Cells 6, 631–640 (2001).
Leng, C. et al. Reduction of graft-versus-host disease by histone deacetylase inhibitor suberonylanilide hydroxamic acid is associated with modulation of inflammatory cytokine milieu and involves inhibition of STAT1. Exp. Hematol. 34, 776–787 (2006).
Imanguli, M.M., Swaim, W.D., League, S.C., Gress, R.E., Pavletic, S.Z. & Hakim, F.T. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 113, 3620–3630 (2009).
Schreiber, S. et al. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut 51, 379–385 (2002).
Capitini, C.M. et al. Absence of STAT1 in donor-derived plasmacytoid dendritic cells results in increased STAT3 and attenuates murine GVHD. Blood 124, 1976–1986 (2014).
Spoerl, S. et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 123, 3832–3842 (2014).
Robb, R.J. et al. Type I-IFNs control GVHD and GVL responses after transplantation. Blood 118, 3399–3409 (2011).
Bossard, C. et al. Plasmacytoid dendritic cells and Th17 immune response contribution in gastrointestinal acute graft-versus-host disease. Leukemia 26, 1471–1474 (2012).
Zhao, K. et al. The identification and characteristics of IL-22-producing T cells in acute graft-versus-host disease following allogeneic bone marrow transplantation. Immunobiology 218, 1505–1513 (2013).
He, S. et al. A new approach to the blocking of alloreactive T cell-mediated graft-versus-host disease by in vivo administration of anti-CXCR3 neutralizing antibody. J. Immunol. 181, 7581–7592 (2008).
Zohar, Y. et al. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J. Clin. Invest. 124, 2009–2022 (2014).
Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Stacey, M.A. et al. Neutrophils recruited by IL-22 in peripheral tissues function as TRAIL-dependent antiviral effectors against MCMV. Cell Host Microbe 15, 471–483 (2014).
Schwab, L. et al. Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat. Med. 20, 648–654 (2014).
Acknowledgements
We acknowledge the technical support of D. Paris and F. Coulon for animal care, and Anna Daoui for her technical help. We also thank the “Agence de Biomédecine”, the Agence Nationale de la Recherche (Labex LipSTIC, ANR-11-LABX-0021; IHU-Cesti, ANR-10-IBHU-005), and the Etablissement Français du Sang (AO#2011-11 and AO#2014-10) for their generous and continuous support to our research work. F.M. was supported by educational grants from the “Association for Training, Education and Research in Hematology, Immunology and Transplantation” (ATERHIT). M.M. thanks Pr JV Melo (University of Adelaide, Australia) for critical reading of the manuscript.
Author contributions
All authors listed on the manuscript have substantially contributed to this work. B.L. participated in experimental design, performed research, analyzed data, and wrote the manuscript. B.G. designed experimental research, interpreted data, and wrote the manuscript. M.C. and C.G. participated in experimental work. J.C.R. generated IL-22- and IFNAR-deficient mice. F.M. and C.B. performed histological analyses. M.M. and P.S. participated in experimental design, interpretation of data, and helped writing and revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Lamarthée, B., Malard, F., Gamonet, C. et al. Donor interleukin-22 and host type I interferon signaling pathway participate in intestinal graft-versus-host disease via STAT1 activation and CXCL10. Mucosal Immunol 9, 309–321 (2016). https://doi.org/10.1038/mi.2015.61
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2015.61
This article is cited by
-
IL-22-dependent dysbiosis and mononuclear phagocyte depletion contribute to steroid-resistant gut graft-versus-host disease in mice
Nature Communications (2021)
-
The good and the bad about separation anxiety: roles of IL-22 and IL-22BP in liver pathologies
Seminars in Immunopathology (2021)