Abstract
Induction of mucosal immunoglobulin-A (IgA) capable of providing a first line of defense against bacterial and viral pathogens remains a major goal of needle-free vaccines given via mucosal routes. Innate immune cells are known to play a central role in induction of IgA responses by mucosal vaccines, but the relative contribution of myeloid cell subsets to these responses has not firmly been established. Using an in vivo model of sublingual vaccination with Bacillus anthracis edema toxin (EdTx) as adjuvant, we examined the role of myeloid cell subsets for mucosal secretory IgA responses. Sublingual immunization of wild-type mice resulted in a transient increase of neutrophils in sublingual tissues and cervical lymph nodes. These mice later developed Ag-specific serum IgG responses, but not serum or mucosal IgA. Interestingly, EdTx failed to increase neutrophils in sublingual tissues and cervical lymph nodes of IKKβΔMye mice, and these mice developed IgA responses. Partial depletion of neutrophils before immunization of wild-type mice allowed the development of both mucosal and serum IgA responses. Finally, co-culture of B cells with neutrophils from either wild-type or IKKβΔMye mice suppressed secretion of IgA, but not IgM or IgG. These results identify a new role for neutrophils as negative regulators of IgA responses.
Similar content being viewed by others
INTRODUCTION
Mucosal surfaces are constantly exposed to microorganisms and represent the main portal of entry of pathogens and toxins. Mucosal immunoglobulin-A (IgA) or secretory IgA (SIgA) neutralizes pathogenic microorganisms and toxins, interferes with bacterial or viral colonization of the epithelium, and participates in homeostasis of mucosal tissues.1 Ideally, vaccines capable of promoting both IgG in the bloodstream and SIgA in mucosal tissues would provide two layers of defense for optimal protection against infectious agents. Injected vaccines containing alum, the most widely used adjuvant, induce serum IgG responses, but unlike experimental mucosal adjuvants, fails to promote SIgA responses.2, 3 Cholera toxin (CT) and the related heat labile toxin (LT) I of Escherichia coli are the most studied experimental adjuvants for induction of SIgA,4 however, their inherent toxicity precludes their use in oral or nasal vaccines.
Cytokines play a crucial role in shaping the profile of T-helper (Th) cytokine responses as well as the Ig isotype and subclass responses. Previous studies have shown that the mucosal adjuvant CT induces pro-inflammatory cytokine (i.e., interleukin-6 (IL-6) or IL-1β) secretion by antigen-presenting cells (i.e., macrophages and dendritic cells).5, 6 CT also induces transforming growth factor beta (TGF-β) and IL-10, two anti-inflammatory cytokines that play a central role in the induction of SIgA.6, 7, 8 Studies with live bacterial and viral vectors as well as immunization studies with Th1-inducing cytokines (i.e., IL-12 and IL-18) have now established that SIgA can also be induced in the context of Th1-biased responses.4 More recently, the ability of CT as an adjuvant to promote SIgA responses was shown to be impaired in mice lacking IL-17A, suggesting a role for IL-17A or related signaling in SIgA responses.6 In this regard, differentiation of Th17 cells requires IL-1β, IL-6, and TGF-β,6, 9 which are cytokines that support IgA responses. Unlike Th1 and Th2 cytokines, which activate JAK–STAT signaling pathways, signaling through IL-17R activates Act1 for subsequent activation of the classical nuclear factor κB (NF-κB) signaling pathway.10 Furthermore, IL-17A directly triggers Ig class switching to IgG2a and IgG3, but not to IgG1.11 To our knowledge, it is still unclear whether production of IgA is directly regulated by IL-17A/IL-17RA signaling in B cells.
The NF-κB pathway plays an important role in inflammatory responses and a number of stimuli can lead to NF-κB translocation to the nucleus.12 Previous studies have shown that the NF-κB pathway can mediate both pro- and anti-inflammatory effects13, 14 depending on the immune cells in which the IKKβ–NF-κB signaling occurs15 and stimuli to which they are exposed. A recent study showed a link between activation of the non-canonical NF-kB pathway in B cells and their ability to undergo immunoglobulin class switch for the production of IgA.16 However, it remains unclear if IKKβ-dependent signaling in myeloid cells regulates IgA responses to mucosal vaccination.
Sublingual tissues have been used as a delivery site for bacterial and viral vaccines,17, 18 and cervical lymph nodes (CLNs) were identified as the primary site of antigen presentation after sublingual immunization.19 However, how innate immune cells in sublingual tissues and/or CLNs regulate antibody production remains unknown. Edema toxin (EdTx) is one of the exotoxins produced by the Gram-positive, spore-forming rod Bacillus anthracis.20 EdTx is composed of two subunits: a binding subunit and an enzymatic subunit. The binding subunit, or protective Ag (PA), allows the binding of these toxins to the anthrax toxin receptors that are expressed by most cells. The enzymatic subunit, or edema factor, is a calmodulin- and calcium-dependent adenylate cyclase that catalyzes the conversion of ATP to cyclic AMP.20, 21 We previously showed that EdTx is a mucosal adjuvant that promotes mucosal and systemic immunity to intranasally co-administered vaccine antigens.22, 23 Here we addressed the contribution of monocytes/macrophages to mucosal SIgA responses after sublingual immunization. Using B. anthracis EdTx as a model of vaccine adjuvant to target anthrax toxin receptors, we show a previously unknown role of neutrophils as negative regulators of IgA responses. Thus, recruitment of neutrophils into sublingual tissues and CLNs shortly after sublingual immunization impaired the development of IgA responses. The negative role of neutrophils in IgA responses was confirmed in vivo by depletion of neutrophils before immunization with EdTx and in vitro by co-culture of B cells with neutrophils.
RESULTS
Toxin adjuvants differentially recruit myeloid-lineage cells into sublingual tissues
Both CT24 and EdTx22, 23 are mucosal adjuvants that promote mucosal SIgA responses via the nasal route. CT can also promote IgA responses when used as an adjuvant for vaccines given by the epicutaneous route or topically on the sublingual mucosa.19, 25 In contrast, EdTx was not effective at inducing IgA when used as an epicutaneous (Duverger et al., unpublished observation) or sublingual adjuvant. To elucidate the mechanism underlying the inability of EdTx to induce IgA by the sublingual route, we first analyzed innate cell subsets present in sublingual tissues after sublingual application of EdTx. The number of CD11b+ myeloid cells increased in the sublingual tissues of mice 3 h after application of EdTx, but not CT (Figure 1a). Flow cytometric26 and morphologic analysis of the myeloid cell subsets (Supplementary Figures S1B–D online) in sublingual tissues 3 h after application of EdTx showed a high frequency (32%) of CD11b+F4/80−Gr-1high cells (neutrophils) and a lower frequency (12%) of CD11b+F4/80+Gr-1− cells (non-inflammatory monocytes). The frequencies of CD11b+F4/80+Gr-1low cells (macrophages or DCs) and CD11b+F4/80+Gr-1high cells (inflammatory monocytes) were not affected by EdTx. In contrast with EdTx, CT increased the frequency (34 vs. 26%) of macrophages/dendritic cell (Figure 1c,d).
Cholera and edema toxin promote different profiles of myeloid cell subsets in sublingual tissues. Sublingual tissues were collected 3 h after sublingual administration of phosphate-buffered saline (PBS), cholera toxin (CT) (2 μg) or edema toxin (EdTx) (15 μg). (a) Flow cytometry analysis of total myeloid cells. Top: absolute number; Bottom: frequency of CD11b+ cells. (b) Gating strategy for identification of myeloid cell subsets (c) Detailed flow cytometry analysis of myeloid cell subsets. (d) Radar plots to summarize the profile of myeloid cell subsets in sublingual tissues. Data are expressed as mean±s.d. (n=3). *P≤0.05 compared with PBS.
EdTx does not recruit neutrophils into sublingual tissues of mice lacking IKKβ in myeloid cells
The adjuvant activities of CT and EdTx involve pro-inflammatory responses and acquisition of antigen-presenting cell functions by myeloid cells.22, 23, 27, 28 The transcription factor NF-κB is a master regulator of cytokine responses and migration of innate cells.29 We previously showed that activation of NF-κB in mouse epithelial cells lacking IKKβ and with impaired ability for nuclear translocation of phospoNF-κB p65, resulted in increased pSTAT3 responses in gut tissues.30 Thus, we examined how EdTx affects the expression of STAT3 in sublingual tissues of control and IKKβΔMye mice, which lack IKKβ in myeloid cells. As depicted in Figure 2a and Supplementary Figure S2, pSTAT3 levels were low in tissues of mice that received saline and increased after application of EdTx. In contrast, pNF-κB levels were higher in tissues of phosphate-buffered saline- than in EdTx-treated mice. Although the transcription factors appeared to be regulated in the opposite direction after EdTx-treatment, the difference in their levels of expression failed to reach statistical significance during the time frame analyzed (Figure 2a and Supplementary Figure S2).
Expression of phospho-NF-κB, phospho-STAT3, and profile of myeloid cell subsets in sublingual tissues after application of edema toxin (EdTx). Sublingual tissues were collected at different time points after sublingual administration of phosphate-buffered saline (PBS) or EdTx (15 μg). (a) A representative Western-blot picture (from three independent experiments) of β-actin and overall (cytoplasmic and nuclear) phospho-NF-κB p65 (pNF-κB p65), and pSTAT3 levels in sublingual tissues 1 and 2 h after administration of EdTx. (b, c) Flow cytometry analysis of myeloid cell subsets 3 and 6 h after sublingual application of EdTx. All data are expressed as mean±s.d. (n=3). *P≤0.05 compared with PBS, and ▾P≤0.05 compared with C57BL/6.
We also analyzed myeloid cells in sublingual tissues at 3 and 6 h after application of EdTx (Figure 2b,c and Supplementary Figure S3). Unlike control C57BL/6 mice, the IKKβΔMye mice did not exhibit an important increase in the frequency of CD11b+ cells in the sublingual tissues 3 h after application of EdTx (Figure 2b). Control C57BL/6 and IKKβΔMye mice exhibited a similar proportion of myeloid cell subsets before application of EdTx, except for non-inflammatory monocytes, which were higher in IKKβΔMye than in control C57BL/6 mice (Figure 2c and Supplementary Figure S3). Three hours after application of EdTx, sublingual tissues of IKKβΔMye mice showed significantly lower frequencies of neutrophils when compared with C57BL/6 (Figure 2c and Supplementary Figure S3).
EdTx was reported to differentially affect the recruitment and cytokine secretion by some immune cells.31 Therefore, we also examined the expression of CCL2 and CXCL2, two chemokines known to recruit inflammatory monocytes and neutrophils, respectively. Sublingual tissue cells of control C57BL/6 and IKKβΔMye mice had similar basal levels of CCL2 and CXCL2 messenger RNA (mRNA), and exhibited similar kinetics and magnitude of responses after exposure to EdTx (Supplementary Figure S4). We also examined the expression of CCR2 and CXCR2, the receptors of CCL2 and CXCL2 by myeloid cell subsets, in sublingual tissues 3 h after application of EdTx in vivo (Figure 3). Since leukotriene B4 could mediate chemotaxis of macrophages and granulocytes,32, 33 the expression of the leukotriene B4 receptor (LTB4R2) was also investigated. Neutrophils in sublingual tissues of C57BL/6 and IKKβΔMye mice exhibited similar profiles of receptor expression (Figure 3b,c). On the other hand, macrophages/DCs and non-inflammatory monocytes collected in sublingual tissues of IKKβΔMye mice exhibited higher frequencies of CCR2+, CXCR2+, and LTB4R2+ cells. Alone, these results cannot explain the higher number of neutrophils in the sublingual tissues of C57BL/6. The pie diagram (Figure 3c), which summarizes the relative contribution of each receptor in myeloid cell subsets shows a broader profile of receptor expression in macrophages/DCs and non-inflammatory monocytes of IKKβΔMye mice. Thus, these cells may have a competitive advantage for responding to chemoattractant signals via ligand binding to these receptors.
Expression of chemokine and leukotriene B4 receptors by myeloid cell subsets in sublingual tissues. Sublingual tissues were collected 3 h after sublingual application of phosphate-buffered saline (PBS) or edema toxin (EdTx) (15 μg). Expression of CCR2, CXCR2, and LTB4R2 by myeloid cell subsets was analyzed by flow cytometry. (a) Gating strategy for identification of chemoattractant receptors on myeloid cell subsets. (b) Percentage of receptor-positive cells. (c) Pie diagrams of relative expression of individual receptors were generated using the formula: Relative number=(% positive cells for a receptor × 1/sum of % positive cells for all receptors) × 100. Data are expressed as mean±s.d. (n=4). *P≤0.05 receptor expression compared with C57BL/6 mice and ▾P≤0.05 compared with other receptors in the same group.
IKKβ deficiency in myeloid cells enhances the adjuvant activity of EdTx for sublingual immunization and promotes Ag-specific SIgA responses
We next asked whether the broader expression of chemokine receptors and LTB4R2s by macrophage/dendritic cell and non-inflammatory monocytes in sublingual tissues of IKKβΔMye mice after application of EdTx could affect the profile of immune responses induced by this adjuvant. For this purpose, mice were immunized via the sublingual route with recombinant Yersinia pestis F1-V antigen and B. anthracis EdTx as adjuvant. Sublingual co-application of EdTx enhanced antigen (F1-V)-specific serum IgG responses (IgG and IgG1) and no difference was seen between control C57BL/6 and IKKβΔMye mice (Figure 4a). The similar levels of IgE responses were seen in control C57BL/6 and IKKβΔMye mice, suggesting that Th2-dependent Abs were not affected in IKKβΔMye mice. On the other hand, IKKβΔMye mice exhibited enhanced IgG2c responses (Figure 4a). Interestingly, unlike control C57BL/6 mice, IKKβΔMye mice developed antigen-specific serum IgA responses, (Figure 4a). The increase in serum IgA responses in IKKβΔMye mice was associated with antigen-specific SIgA in the saliva, vaginal washes, and fecal extracts (Figure 4b). We also asked whether the specificity and function of antibody (Ab) induced by EdTx as sublingual adjuvant were affected by the absence of IKKβ-dependent signaling in myeloid cells. After sublingual immunization with F1-V alone, control C57BL/6 and IKKβΔMye mice developed IgG Abs, which were directed against the same peptide (i.e., P1–17 or P1) of the F1-capsular antigen (Figure 4c and Table 1). EdTx as an adjuvant promoted Abs that reacted to two additional epitope peptides in control C57BL/6 and IKKβΔMye mice. However, only one of the additional peptides (P19) was shared by Abs from control C57BL/6 and IKKβΔMye mice (Figure 4c and Table 1).
Lack of IKKβ signaling in myeloid cells improves the adjuvant activity of edema toxin (EdTx) after sublingual immunization and promotes antigen-specific ecretory immunoglobulin-A (SIgA) responses. Mice were immunized three times at weekly intervals by sublingual application of F1-V alone or F1-V plus EdTx as an adjuvant. Serum, vaginal washes ,and fecal samples were collected 1 week (Day 21), and saliva samples were collected 2 weeks (Day 28) after the last immunization. F1-V-specific Ab responses were analyzed by enzyme-linked immunosorbent assay (ELISA). (a) F1-V-specific serum antibody responses; (b) F1-V-specific SIgA responses in mucosal secretions. The end-point titers were expressed as Log2 GMTs.±s.d. from C57BL/6 (n=5), and IKKβΔMye mice (n=3–5). *P≤0.05 compared with C57BL/6 mice and ▾P≤0.05 compared with group immunized with F1-V alone. (c) F1 epitope-specific serum IgG responses in C57BL/6 (n=5) and IKKβΔMye mice (n=5). Sera were diluted 1:50 (groups immunized with F1-V alone) or 1:500 (groups immunized with F1-V plus EdTx), and IgG responses against linear epitopes of the capsular F1 antigen were analyzed by epitope-specific ELISA. Results were expressed as mean OD405nm±s.d. *P≤0.05 compared with group immunized with F1-V alone.
We also wondered whether the enhanced IgA responses seen in IKKβΔMye mice were restricted to FI-V as antigen and EdTx as adjuvant. Nasal immunization with EdTx is known to promote immunity against the EdTx binding subunit PA.22, 23 Sublingual immunization with EdTx also enhanced PA-specific serum IgG Ab titers in IKKβΔMye mice (Supplementary Figure S5A) and this was consistent with the enhanced levels of PA-specific neutralizing Abs (Supplementary Figure S5B). In addition, we found that serum and mucosal IgA responses induced by CT as a sublingual adjuvant were enhanced in IKKβΔMye mice (Supplementary Figure S6).
Finally, we analyzed antigen (F1-V)-specific Th cytokine responses supported by EdTx as an adjuvant for sublingual vaccination. In wild-type C57BL/6 mice, the sublingual adjuvant EdTx enhanced the frequency of antigen-specific IFN-γ producing Th cells in the spleen (Figure 5). On the other hand, the IKKβΔMye mice exhibited a broader profile of Th-cell responses with a significant increase of antigen-specific IFN-γ (Th1), IL-4 (Th2), IL-10+, and IL-17A+ producing Th cells (Figure 5).
Lack of IKKβ in myeloid cells broadens antigen-specific T-helper cytokine responses to sublingual immunization with edema toxin as an adjuvant. Spleen cells were collected three weeks after the last immunization and cultured for 5 days in the presence of recombinant F1-V (5 μg ml−1). The numbers of CD4+ T cells expressing Th1, Th2, and Th17 cytokines were analyzed by flow cytometry. Data are expressed as mean±s.d. from C57BL/6 (n=4) and IKKβΔMye mice (n=4). *P≤0.05 compared with C57BL/6, and ▾P≤0.05 compared with mice immunized with F1-V alone.
The frequency of neutrophils inversely correlates with production of IgA in CLNs
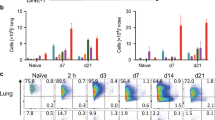
CLNs are considered inductive sites for adaptive immune responses after sublingual19 and nasal34 immunization. We have shown that 6 h after application of EdTx, the frequency of CD11b+ cells returned toward basal levels in sublingual tissues (Figure 2b). We hypothesized that cells had migrated to CLN and thus analyzed myeloid cell subsets in these lymphoid tissues. The frequency of neutrophils was significantly reduced in CLNs of IKKβΔMye compared to control C57BL/6 mice, while the other myeloid cell subsets remained unchanged (Figure 6a). CLN cells from EdTx-treated mice were then cultured in the presence of lipopolysaccharide. Three days later, we found a significantly higher number of IgA-secreting cells in IKKβΔMye than in control C57BL/6 mice (Figure 6b). Of interest, the number of IgA-secreting cells in the CLNs of both control C57BL/6 and IKKβΔMye mice were inversely correlated (r=−0.8) with the numbers of neutrophils in these tissues (Figure 6c).
Inverse correlation between the number of neutrophils and immunoglobulin-A (IgA) responses to sublingual immunization. (a–c) Cervical lymph nodes were collected at 6 h after sublingual administration of edema toxin (EdTx) (15 μg). (a) Flow cytometry analysis of myeloid cell subsets. (b) CLN cells were further cultured for 3 days in the presence of lipopolysaccharide (5 μg ml−1) and Ab-secreting cells analyzed by ELISPOT. (c) Linear-regression models to correlate the frequency of neutrophils and number of Ig isotype-secreting cells. Data are expressed as mean±s.d. (n=4). *P≤0.05 compared with C57BL/6. (d) Wild-type C57BL/6 mice were treated by IP administration of the neutrophil Ly6G-specific 1A8 monoclonal Ab (1A8+C57BL/6 mice). Two days later, control C57BL/6 and IKKβΔMye mice were immunized three times at weekly intervals by sublingual application of F1-V plus EdTx. F1-V-specific IgA Ab responses in serum and fecal samples were analyzed by enzyme-linked immunosorbent assay (ELISA) and end-point titers were expressed as Log2GMTs.±s.d. *P≤0.05 compared with C57BL/6 in each day (n=5).
Reduction of neutrophils augments the adjuvant effect of EdTx on Ag-specific IgA responses
To further establish that an inverse correlation exists between the frequency of neutrophils in sublingual tissues and CLNs, and antigen-specific IgA responses, wild-type C57BL/6 were injected (IP) with a neutrophil Ly6G-specific 1A8 monoclonal Ab 2 days before sublingual immunization with F1-V and EdTx as an adjuvant. This treatment reduced the frequency of neutrophils in CLNs of wild-type C57BL/6 mice (Supplementary Figure S7A), and C57BL/6 mice pre-treated with 1A8 (1A8+C57BL/6) contained virtually no neutrophils in sublingual tissues after application of EdTx (Supplementary Figure S7B). These 1A8+C57BL/6 mice also gradually developed F1-V-specific serum IgA titers over the time points tested and reached higher serum IgA titers than non-treated C57BL/6 or IKKβΔMye mice at Day 28 (Figure 6d). Interestingly, depletion of neutrophils also enhanced mucosal IgA Ab responses; 1A8+C57BL/6 mice produced high levels of F1-V-specific fecal IgA Abs, which were comparable to those measured in IKKβΔMye mice (Figure 6d).
Neutrophils suppress production of IgA by B cells
Our results clearly show that the ability to generate IgA responses is enhanced in the absence of IKKβ in myeloid cells or when the number of neutrophils is reduced. In addition, the ability of EdTx to induce systemic and mucosal IgA responses in IKKβΔMye mice is associated with increased Th17 responses and production of IL-17A (Figure 5). Thus, we next examined how alteration of canonical NF-κB-mediated signaling via IKKβ-deletion in myeloid cells (IKKβΔMye) could support Ig class switch and Ab production by B cells. For this purpose, CD11b-depleted spleen cells from C57BL/6 mice were co-cultured with 20% autologous CD11b+ cells (C57BL/6 CD11b+) or CD11b+ cells from IKKβΔMye mice (IKKβΔMye CD11b+) with or without EdTx in the presence of lipopolysaccharide.35 After 5 days of culture, cells were segregated into IL-17RAlow and IL-17RAhigh cells (Supplementary Figure S8A). Co-culture with IKKβΔMye CD11b+ cells significantly increased the frequency of B220+IL-17RAhigh B cells (Supplementary Figure S8B). As shown in Figure 7a, these cultures contained low frequencies of surface IgA cells among IL-17RAlow B cells regardless of the presence of IKKβΔMye CD11b+ cells. Interestingly, high frequencies of IL-17RAhigh B cells expressed surface IgA and co-culture with IKKβΔMye CD11b+ cells further increased these frequencies. To further elucidate signals that supported IgA responses, we analyzed mRNA levels of the B-cell activators a proliferation-inducing ligand (APRIL) and B-cell activation factor of the TNF family (BAFF), and activation-induced deaminase (AID). Addition of EdTx to cultures of spleen cells enhanced mRNA levels of APRIL, BAFF, and AID, and the presence of IKKβΔMye CD11b+ further enhanced BAFF- and AID-specific mRNA expression (Supplementary Figure S8C). The presence of IKKβΔMye CD11b+ did not affect EdTx-induced IL-1β and IL-6 mRNA levels, but increased EdTx-induced TNF-α, IL-23, IL-10, and Caspase-1 mRNA levels (Supplementary Figure S9). Taken together, these results show that myeloid cells lacking IKKβ provide a microenvironment favorable for Ig class switch and B-cell production of IgA.
Neutrophils suppress the production of immunoglobulin-A (SIgA) by B cells (a) CD11b− spleen cells from C57BL/6 mice were co-cultured with autologous CD11b+ cells from C57BL/6 mice or heterologous CD11b+IKKβΔMye cells in the presence of lipopolysaccharide (LPS; 5 μg ml−1) with or without edema toxin (EdTx; 2 μg ml−1). The frequencies of expression of IgA+ among B220+IL-17RAlow and B220+IL-17RAhigh subpopulations were analyzed by flow cytometry after 5 days of co-culture with autologous or heterologous CD11b+ cells. Data are expressed as mean±s.d. (n=4). *P≤0.05 compared with C57BL/6 CD11b+ cells. (b, c) CD19+ splenocytes from C57BL/6 mice were incubated overnight with 100 ng ml−1 of LPS, washed extensively and then co-cultured with autologous neutrophils from C57BL/6 mice or heterologous neutrophils from IKKβΔMye mice without additional stimuli. (b) IgM, IgG, and IgA levels in 5-day culture supernatants as determined by enzyme-linked immunosorbent assay. Data are expressed as mean±s.d. (n=4). *P≤0.05 compared with CD19+ B cells cultured alone. (c, d) Messenger RNA (mRNA) levels of IgA heavy chain determined by real-time reverse transcriptase-PCR after 24 h of co-culture. (c) Individual mRNA levels in three independent experiments. (d) Relative mRNA levels from co-culture of B cells with neutrophils as a percentage of mRNA levels in cultures of CD19+ B cells alone (n=3). *P≤0.05 compared with CD19+ B cells cultured alone.
To gain insight into the mechanism of how neutrophils affect IgA responses, B cells from C57BL/6 mice were co-cultured with or without neutrophils from C57BL/6 or IKKβΔMye mice for 5 days. The addition of neutrophils from either C57BL/6 or IKKβΔMye mice to cultures of B cells did not affect the secretion of IgM or IgG Abs into culture supernatants (Figure 7b). Interestingly, co-culture with neutrophils significantly reduced the amounts of IgA Abs secreted by B cells and this inhibitory effect was independent of the presence of functional IKKβ in neutrophils (Figure 7b). Finally, mRNA analysis of B cells co-cultured with neutrophils showed that neutrophils reduced the level of IgA heavy chain transcripts in B cells (Figure 7c).
DISCUSSION
Recent studies have identified sublingual immunization as a potentially safer alternative to nasal immunization. However, inductive sites for generating immune responses to sublingual immunization, the identity and function of the cells involved, and the signaling pathways for induction of SIgA via this mucosal route are poorly understood. Here, we show that the ability of a sublingual vaccine to mount a SIgA response inversely correlates with the presence of neutrophils in sublingual tissue and CLNs. We also show that depletion of Ly6G+ cells improves the development of IgA responses after sublingual immunization and that neutrophils impair the transcription of IgA heavy chain in B cells. This work also shows that myeloid cells lacking IKKβ-dependent NF-κB signaling provide an environment that supports the production of IgA by B cells.
Alum is the most widely used adjuvant for injected vaccines. However, attempts to include alum in mucosal vaccines aimed at prompting SIgA responses have been unsuccessful because this adjuvant fails to effectively induce IgA.2 Studies that addressed mechanisms underlying the adjuvant activity of alum have shown that alum acts via Gr-1 splenic myeloid cells expressing IL-4 to stimulate early B-cell priming.36 Other studies have shown that the NALP3 inflammasome was a crucial element in the adjuvant activity of alum by promoting the maturation of inflammatory cytokines37; and furthermore, alum recruits inflammatory monocytes.38 In other studies, intranasal co-administration of human neutrophil proteins enhanced antigen-specific serum IgG responses, but failed to promote SIgA responses.39 These reports are consistent with our finding that less recruitment of neutrophils into sublingual tissues and CLNs of IKKβΔMye mice is a reliable indication of the ability of EdTx as an adjuvant to promote SIgA responses. Because IgG production is not impaired by the recruitment of neutrophils, it is unlikely that neutrophils limit SIgA responses by limiting antigen access to antigen-presenting cells or interactions between the latter and T cells as was previously suggested.40 Induction of SIgA is well-known to require priming of effector cells in unique inductive sites.4 Thus, our finding, that the low proportion of Gr-1+ inflammatory monocytes and/or higher proportion of Gr-1− non-inflammatory monocytes in the sublingual tissue correlates with induction of broad Ab responses consisting of both serum IgG and SIgA responses, is in agreement with the recent report that neutrophils also control the spread of T-cell responses to distant lymph nodes.41 The Gr-1− monocytes, also described as tissue resident myeloid cells, have been classified as alternatively activated macrophages (M2 macrophages) capable of producing IL-10 and TGF-β.26, 42 Interestingly, these two cytokines are central for Ig class switch in B cells and for production of IgA.
Experiments using IKKβΔMye mice provided new insights into signaling for the induction of SIgA responses. Previous studies have shown that the NF-κB pathway can mediate both pro- and anti-inflammatory effects.13, 14 Our data suggest that activation of IKKβ–NF-κB signaling in myeloid cells may in fact reduce their capacity to help B cells undergo Ig class switch for production of IgA. This finding is interesting in light of the recent report that the kinase TBK1 in B cells limits IgA class switch by negative regulation of the non-canonical NF-κB pathway.16 Thus, stimulation of non-canonical NF-κB signaling either directly in B cells or in other antigen-presenting cells could represent a major pathway for induction of IgA Abs. In this regard, we have recently shown that IKKβ deficiency in intestinal epithelial cells increases IgA responses induced by CT used as an oral adjuvant.30 The notion that IKKβ can reduce or suppress the functions of macrophages or DCs is consistent with previous studies by others, suggesting that IKKβ may suppress activation of M1 macrophages during infections through inhibition of STAT-1.15 In those studies, deletion of IKKβ in macrophages increased STAT-1 activation and promoted a shift toward the M1 phenotype, characterized by increased production of pro-inflammatory and inflammatory cytokines, i.e., IL-1β, TNF-α, IL-12 and IFN-γ, and iNOS in response to IP injection of Group B streptococcus or E. coli lipopolysaccharide.13, 15 While our studies showed enhanced antigen-specific Th1 cytokine responses in IKKβΔMye mice after sublingual immunization, the most striking observation was the enhanced IL-17 response.
The IKKβΔMye mice were useful tools that helped us identify the repressive effect of neutrophils on IgA responses. Analysis of chemokine receptors expression by myeloid cell subsets in sublingual tissues revealed a broader expression of CCR2, CXCR2, and LTB4R2 on macrophages/dendritic cell and non-inflammatory monocytes from IKKβΔMye mice. One can speculate that this pattern of receptor expression could improve cellular responses to corresponding ligands and facilitate migration to inductive sites and support IgA responses. Previous studies have shown that injection of alum recruits neutrophils and induces the formation of nodules consistent with those of extracellular DNA traps.43 A recent report showed that the formation of neutrophil extracellular traps requires phosphorylation of p65 NFκB.44 However, neutrophil extracellular traps are primarily known to be involved in the killing of pathogens.45 Furthermore, our results showing that neutrophils from both wild-type and IKKβΔMyemice suppress transcription of IgA heavy chain suggest the involvement of other mechanisms, which will be addressed in future studies.
Nasal immunization with the cyclic AMP-inducing adjuvant CT6 or E.coli heat LT-I46 promotes Th17 responses. Here, we show that EdTx as a sublingual adjuvant promotes antigen-specific Th17 responses in spleen, and is associated with in vitro induction of IL-1β and IL-6. The hallmark cytokine produced by Th17 cells is IL-17A.47 Unlike most Th-derived cytokines, IL-17A does not activate JAK–STAT,10 but engages Act1 leading to activation of IKKβ and downstream NF-κB, C/EBP, and AP-1, which in turn lead to expression of pro-inflammatory cytokines.48, 49 Recently, it has been suggested that Th17 cells stimulate B-cell proliferation and Ig class switch for enhanced Ab production.11 We have shown that CD11b+ cells from IKKβΔMye mice increase specific B-cell populations, i.e., IL-17RAhigh B cells, and that the IL-17RAhigh B cells express higher levels of surface IgA. IL-17A was reported to act as a helper for the development of germinal centers.50 Our results suggest that IKKβΔMye cells stimulate B cells to be more responsive to IL-17A. This pathway could be one of the mechanisms that rescues the mucosal adjuvant EdTx and the induction of SIgA Abs.
The limited understanding of molecular and cellular mechanisms that regulate IgA responses has hampered the development of safe mucosal vaccines capable to promote mucosal IgA production. Using an experimental vaccine adjuvant that does not normally induce SIgA after sublingual immunization, we showed that IKKβ is one of the key regulatory pathways for induction of SIgA responses by sublingual vaccines. We also showed that neutrophils negatively regulate IgA production by B cells, an effect that can be countered by Gr-1− myeloid cells lacking a functional IKKβ. Our results provide new insights for the development of sublingual vaccines that can promote both IgA at mucosal surfaces and IgG in the blood stream for optimal protection against infectious agents.
METHODS
Mice. Control C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) or NCI-Frederick (Frederick, MD) and acclimated to our facility for at least 2 weeks before being used. IKKβΔMye mice were kindly provided by Dr Karin (University of California at San Diego) and were generated by crossing LysMCre mice, expressing Cre downstream of the lysozyme promoter in myeloid-lineage cells, with IKKβf/f mice harboring a loxP-flanked IKKβ gene13, 14 Mice were bred in our facility, maintained in a pathogen-free environment and were used at 8–12 weeks of age. All experiments were performed in co-housed mice in accordance with both NIH and Institutional Animal Care and Use Committee guidelines.
Immunization and sample collection. The F1-V antigen and B. anthracis PA and edema factor were obtained from BEI Resources (Manassas, VA). Mice were immunized three times, i.e., Days 0, 7, and 14 by sublingual application of 30 μl of phosphate-buffered saline containing 50 μg of F1-V antigen alone, or 50 μg of F1-V antigen plus 15 μg EdTx, i.e., 15 μg PA and 15 μg edema factor. Blood and external secretions (fecal extracts, vaginal washes, and saliva) were collected as previously described.24 In selected experiments, mice were injected IP with 0.5 mg of the neutrophil Ly6G-specific 1A8 monoclonal Ab (BioXCell, West Lebanon, NH) 2 days before the sublingual immunization. Neutrophils were isolated from bone marrow and blood using a 62% Percoll gradient, followed by magnetic activated cell sorting-sorting with the aid of CD11b microbeads.
Other materials and methods. Other methods were previously reported and are summarized in the Supplementary Materials. These methods include enzyme-linked immunosorbent assay,22, 23 enzyme-linked immunospot assay,24 macrophage toxicity assay,22, 23 in vitro cultures and flow cytometry analysis, and quantitative real-time reverse transcriptase-PCR.22, 30
References
Macpherson, A. J., McCoy, K. D., Johansen, F. E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
Jackson, E. M. & Herbst-Kralovetz, M. M. Intranasal vaccination with murabutide enhances humoral and mucosal immune responses to a virus-like particle vaccine. PLoS One 7, e41529 (2012).
Lo, D. D., Ling, J. & Eckelhoefer, A. H. M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses. BMC Biotechnol. 12, 7 (2012).
Boyaka, P. N., McGhee, J. R., Czerkinsky, C. & Mestecky, J. Mucosal vaccines: an overview In Mucosal Immunology Vol. 1 (Mestecky, J. ed 855–874 Elsevier, Acadamic Press: San Diego, CA, (2005).
Cong, Y., Oliver, A. O. & Elson, C. O. Effects of cholera toxin on macrophage production of co-stimulatory cytokines. Eur. J. Immunol. 31, 64–71 (2001).
Datta, S. K. et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl Acad. Sci. USA 107, 10638–10643 (2010).
Defrance, T., Vanbervliet, B., Briere, F., Durand, I., Rousset, F. & Banchereau, J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J. Exp. Med. 175, 671–682 (1992).
Kim, P. H., Eckmann, L., Lee, W. J., Han, W. & Kagnoff, M. F. Cholera toxin and cholera toxin B subunit induce IgA switching through the action of TGF-beta 1. J. Immunol. 160, 1198–1203 (1998).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Gaffen, S. L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9, 556–567 (2009).
Mitsdoerffer, M. et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl Acad. Sci. USA 107, 14292–14297 (2010).
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009).
Greten, F. R. et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130, 918–931 (2007).
Lawrence, T., Gilroy, D. W., Colville-Nash, P. R. & Willoughby, D. A. Possible new role for NF-kappaB in the resolution of inflammation. Nat. Med. 7, 1291–1297 (2001).
Fong, C. H. et al. An antiinflammatory role for IKKbeta through the inhibition of ‘classical’ macrophage activation. J. Exp. Med. 205, 1269–1276 (2008).
Jin, J. et al. The kinase TBK1 controls IgA class switching by negatively regulating noncanonical NF-kappaB signaling. Nat. Immunol. 13, 1101–1109 (2012).
Raghavan, S. et al. Sublingual immunization protects against Helicobacter pylori infection and induces T and B cell responses in the stomach. Infect. Immun. 78, 4251–4260 (2010).
Song, J. H. et al. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl Acad. Sci. USA 105, 1644–1649 (2008).
Song, J. H. et al. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. J. Immunol. 182, 6851–6860 (2009).
Moayeri, M. & Leppla, S. H. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 7, 19–24 (2004).
Tang, W. J. & Guo, Q. The adenylyl cyclase activity of anthrax edema factor. Mol. Aspects Med. 30, 423–430 (2009).
Duverger, A. et al. Contributions of edema factor and protective antigen to the induction of protective immunity by Bacillus anthracis edema toxin as an intranasal adjuvant. J. Immunol. 185, 5943–5952 (2010).
Duverger, A. et al. Bacillus anthracis edema toxin acts as an adjuvant for mucosal immune responses to nasally administered vaccine antigens. J. Immunol. 176, 1776–1783 (2006).
Boyaka, P. N. et al. Chimeras of labile toxin one and cholera toxin retain mucosal adjuvanticity and direct Th cell subsets via their B subunit. J. Immunol. 170, 454–462 (2003).
Cuburu, N. et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 25, 8598–8610 (2007).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Bromander, A. K., Kjerrulf, M., Holmgren, J. & Lycke, N. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand. J. Immunol. 37, 452–458 (1993).
McGee, D. W., Beagley, K. W., Aicher, W. K. & McGhee, J. R. Transforming growth factor-beta and IL-1 beta act in synergy to enhance IL-6 secretion by the intestinal epithelial cell line, IEC-6. J. Immunol. 151, 970–978 (1993).
Grivennikov, S. I. & Karin, M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 21, 11–19 (2010).
Bonnegarde-Bernard, A. et al. IKKbeta in intestinal epithelial cells regulates allergen-specific IgA and allergic inflammation at distant mucosal sites. Mucosal Immunol. 7, 257–267 (2014).
Klezovich-Benard, M. et al. Mechanisms of NK cell-macrophage Bacillus anthracis crosstalk: a balance between stimulation by spores and differential disruption by toxins. PLoS Pathog. 8, e1002481 (2012).
Grespan, R. et al. CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum. 58, 2030–2040 (2008).
Chou, R. C. et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity 33, 266–278 (2010).
Kraal, G. Nasal-associated lymphoid tissue In Mucosal Immunology Vol 1 (Mestecky, J., Lamm, M.E., Strober, W., Bienenstock, J., McGhee, J.R. & Mayer, L. eds 415–422 Elsevier, Academic Press: San Diego, CA, (2005).
Lycke, N. & Strober, W. Cholera toxin promotes B cell isotype differentiation. J. Immunol. 142, 3781–3787 (1989).
Jordan, M. B., Mills, D. M., Kappler, J., Marrack, P. & Cambier, J. C. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science 304, 1808–1810 (2004).
Eisenbarth, S. C., Colegio, O. R., O'Connor, W., Sutterwala, F. S. & Flavell, R. A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008).
Kool, M. et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 205, 869–882 (2008).
Lillard, J. W. Jr, Boyaka, P. N., Chertov, O., Oppenheim, J. J. & McGhee, J. R. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl Acad. Sci. USA 96, 651–656 (1999).
Yang, C. W., Strong, B. S., Miller, M. J. & Unanue, E. R. Neutrophils influence the level of antigen presentation during the immune response to protein antigens in adjuvants. J. Immunol. 185, 2927–2934 (2010).
Yang, C. W. & Unanue, E. R. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J. Exp. Med. 210, 375–387 (2013).
Martinez, F. O., Helming, L. & Gordon, S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 (2009).
Munks, M. W. et al. Aluminum adjuvants elicit fibrin-dependent extracellular traps in vivo. Blood 116, 5191–5199 (2010).
Lapponi, M. J. et al. Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 345, 430–437 (2013).
Branzk, N. & Papayannopoulos, V. Molecular mechanisms regulating NETosis in infection and disease. Semin. Immunopathol. 35, 513–530 (2013).
Brereton, C. F., Sutton, C. E., Ross, P. J., Iwakura, Y., Pizza, M., Rappuoli, R. et al. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. J. Immunol. 186, 5896–5906 (2011).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Maitra, A. et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc. Natl Acad. Sci. USA 104, 7506–7511 (2007).
May, M. J. IL-17R signaling: new players get in on the Act1. Nat. Immunol. 12, 813–815 (2011).
Hsu, H. C. et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 9, 166–175 (2008).
Acknowledgements
Supported by grants from the National Institutes of Health (R01 AI043197 and R01 AT006552 to PNB), and a fellowship from Fondation Lelous, France (to AB-B). We thank Dr Michael Karin for providing IKKβ-deficient mice and Dr Kate Hayes-Ozello for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Jee, J., Bonnegarde-Bernard, A., Duverger, A. et al. Neutrophils negatively regulate induction of mucosal IgA responses after sublingual immunization. Mucosal Immunol 8, 735–745 (2015). https://doi.org/10.1038/mi.2014.105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2014.105
This article is cited by
-
Is the oral microbiome a source to enhance mucosal immunity against infectious diseases?
npj Vaccines (2021)
-
Different types of adjuvants in prophylactic and therapeutic human papillomavirus vaccines in laboratory animals: a systematic review
Archives of Virology (2020)
-
A Novel Supplementation Approach to Enhance Host Response to Sublingual Vaccination
Scientific Reports (2019)