Abstract
Inflammation of human bronchial epithelia (HBE) activates the endoplasmic reticulum (ER) stress transducer inositol-requiring enzyme 1 (IRE1)α, resulting in IRE1α-mediated cytokine production. Previous studies demonstrated ubiquitous expression of IRE1α and gut-restricted expression of IRE1β. We found that IRE1β is also expressed in HBE, is absent in human alveolar cells, and is upregulated in cystic fibrosis and asthmatic HBE. Studies with Ire1β−/− mice and Calu-3 airway epithelia exhibiting IRE1β knockdown or overexpression revealed that IRE1β is expressed in airway mucous cells, is functionally required for airway mucin production, and this function is specific for IRE1β vs. IRE1α. IRE1β-dependent mucin production is mediated, at least in part, by activation of the transcription factor X-box binding protein-1 (XBP-1) and the resulting XBP-1-dependent transcription of anterior gradient homolog 2, a gene implicated in airway and intestinal epithelial mucin production. These novel findings suggest that IRE1β is a potential mucous cell-specific therapeutic target for airway diseases characterized by mucin overproduction.
Similar content being viewed by others
Introduction
Airway diseases such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis (CF) are characterized by chronic inflammation and hypersecretion of mucins. CF and chronic obstructive pulmonary disease airway epithelia exhibit increased secretion of the major polymeric mucins MUC5B and MUC5AC.1 In addition, MUC5AC, a low-charge glycoform of MUC5B, and MUC2 are the major secretory mucins in asthmatic patients.2 Increased mucin production occurs in airways in specialized goblet cells that act in concert with other mucosal innate defense mechanisms, e.g., ciliated cell-driven mucociliary clearance and macrophage activation, to restore airway homeostasis after a noxious insult. Failure to return mucin production to normal levels can lead to chronic pulmonary disease. As a complete knowledge of the pathways responsible for mucin overproduction in pulmonary diseases is lacking, understanding these pathways could lead to novel treatments.
Previous studies have shown that a form of endoplasmic reticulum (ER) stress, the unfolded protein response (UPR), is activated in inflamed airway epithelia.3, 4, 5, 6 The increased demand for new, unfolded inflammatory mediators and defensive factors is thought to alter ER homeostasis and trigger the UPR.4, 7, 8, 9, 10 Eukaryotic cells exhibit three major UPR pathways: (1) inositol-requiring enzyme 1 (IRE1), (2) activating transcription factor 6 (ATF6), and (3) PKR-like ER kinase/pancreatic eIF2α kinase (PERK).11 IRE1 (the focus of the present study) is a transmembrane ER stress sensor that exists in two isoforms in mammals, α and β (also known as ER to nucleus signaling 1 and 2, ERN1 and ERN2, respectively). Activation of IRE1 results in dimerization, trans-autophosphorylation, and activation of its C-terminal endoribonuclease activity,8, 10 leading to removal of a 26 nucleotide intron from the leucine zipper transcription factor XBP-1 mRNA.12, 13 The spliced XBP-1 mRNA is translated into a potent transcription factor,12, 13 which upregulates genes encoding ER chaperones involved in protein folding.9, 14
To date, most studies have investigated the relevance of UPR-dependent IRE1 signaling by focusing on IRE1α. However, IRE1α and IRE1β show a marked difference in tissue expression, which has functional significance. For instance, IRE1α is ubiquitously expressed and Ire1α−/− mice exhibit embryonic lethality.15, 16 In contrast, IRE1β expression has been reported only in gastrointestinal epithelium.17 Although Ire1β−/− mice are viable, they are more susceptible to dextran sodium sulfate (DSS)-induced colitis than wild-type (WT) mice.17 Loss of the intestinal mucin Muc2 also leads to increased sensitivity of mice to DSS-induced colitis,18 suggesting a functional link between IRE1β expression and mucin production.
During airway epithelial inflammation, previous studies have shown that a key consequence of IRE1 activation-dependent XBP-1 mRNA splicing is the expansion of ER Ca2+ stores in ciliated cells, which provides a mechanism for amplification of Ca2+-dependent cytokine secretion.3, 4, 5 However, no study has addressed the role of IRE1 signaling in airway mucous cell function, e.g., mucin production, despite the fact that mucin overproduction requires increased protein folding and upregulation of the secretory capacity, which could all be regulated by the UPR.
As the development of the gut and respiratory tracts initiates from a single foregut tube, we hypothesized that IRE1β might also be functionally expressed in airways. Furthermore, we hypothesized that the airway and gut-specific expression of IRE1β is related to the requirement for both tissues to produce mucins via specialized mucous cells. The present studies demonstrate mucous cell-specific expression of IRE1β in both mouse and human airways. IRE1β is required for mucin production and this function is mediated by activation of XBP-1-dependent transcription of anterior gradient homolog 2 (AGR2), a gene implicated in airway and intestinal epithelial mucin production. These findings suggest that IRE1β represents a novel, mucous cell-specific, therapeutic target for chronic airway diseases typified by overproduction of mucins.
Results
IRE1β is expressed in respiratory epithelium and is associated with genes involved in mucin/mucus production
As the UNIGENE (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.592041) data suggested that IRE1β is expressed in airways, we directly investigated the expression of IRE1β/Ire1β and IRE1α/Ire1α by non-quantitative reverse transcriptase-PCR analyses in several mouse and human tissues, including various samples from the respiratory tract. Figure 1a illustrates the Ire1β expression in respiratory (nasopharynx, trachea, and bronchus) and gastrointestinal (stomach and colon) tissues of mice. Expression of Ire1β was not found in murine lung parenchyma (Figure 1a). In contrast, Ire1α was expressed in all tissues studied, albeit its lowest expression was found in the heart (Figure 1a). Quantitative reverse transcriptase-PCR analyses (relative to nasopharynx) confirmed the differential expression of the IRE1 isoforms in the murine tissues (Figure 1b).
Expression of inositol-requiring enzyme-1 (IRE1)β in murine and human tissues. (a) Reverse transcriptase (RT)-PCR illustrating Ire1β (top panel) and Ire1α (middle panel) expression in murine tissues. Expression of ribosomal 18S gene (bottom panel) was used as an internal control. (b) Quantitative RT-PCR of IRE1β and IRE1α expression in the same murine tissues shown in a. (c) RT-PCR depicting IRE1β expression in human bronchial airway epithelia (HBE) and human colon (positive control). (d) RT-PCR of IRE1α and IRE1β from freshly isolated human alveolar type II cells. Human alveolar type II cells do not express IRE1β (colonic tissue was used as a positive control). (e) Differentiation of primary cultures of HBE associated with the mucous phenotype couples to increased levels of IRE1β mRNA.
IRE1β is expressed in primary cultures of well-differentiated human bronchial epithelia (HBE) and human gut (Figure 1c), but as predicted from the findings in mice IRE1β mRNA expression was not seen in purified preparations of human alveolar type II cells (Figure 1d).
Further analyses of expression data using Celsius,19 a Gene Expression Tool (UGET; http://genome.ucla.edu/projects/UGET), which calculates a correlation matrix for gene expression across many available sample sets from many cell types, revealed that IRE1β/Ire1β, but not IRE1α/Ire1α, exhibits a strong correlation with several genes involved in mucous cell function and mucus production in human and mice (Supplementary Material online and Supplementary Table 1 online). Among these genes, a very strong correlation of IRE1β/Ire1β gene expression was found with SAM pointed domain containing ETS transcription factor (SPDEF), which regulates genes associated with mucus production and is important for pulmonary goblet cell differentiation.20 IRE1β/Ire1β gene expression was also strongly associated with AGR2, a protein disulfide isomerase essential for production of intestinal mucus22 and airway epithelial mucins,23 and CLCA1/3 (GOB-5), a member of the calcium-activated chloride channel family induced in allergic airways, and strongly associated with mucin gene regulation and mucous cell transdifferentiation24, 25 (Supplementary Material online and Supplementary Table 1 online). Other genes highly correlated with IRE1β/Ire1β gene expression included several enzymes predicted to be involved in mucin glycosylation, e.g., the sulfo- and glucosaminyl-transferase enzymes (Supplementary Material online and Supplementary Table 1 online). This same set of genes was not correlated with the expression of IRE1α/Ire1α.
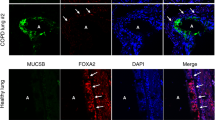
The Celsius data suggested a link between the IRE1β expression and airway epithelial mucin production, and we utilized primary cultures of HBE to further address this link in a highly relevant model. The appearance of mucins in polarized primary cultures of HBE begins ∼7–14 days after the initiation of culture, before the epithelium begins to express a ciliated phenotype.26, 27 Using this model, a strong correlation was identified between the mRNA levels of IRE1β and MUC5B, the major mucin secreted by mucous cells in primary cultures on HBE,26, 27 and between the mRNA levels of IRE1β and AGR2 (Figure 1e). Notably, the mRNA levels of SPDEF were the least upregulated in this physiological model of HBE differentiation (Figure 1e).
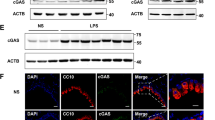
We next addressed by immunohistochemical and mRNA analyses the localization and expression levels of IRE1β protein in native human tissues from airway diseases characterized by increased mucous cell expression. In non-inflamed normal and inflamed CF HBE, the staining suggested that IRE1β expression was increased in CF as compared with normal epithelia and, importantly, that IRE1β localized in goblet/mucous cells of CF epithelia, i.e., in cells exhibiting “goblet” morphology and containing mucin granules, which are hallmarks of mucin-producing cells in human CF airway epithelia (Figure 2a). Notably, the ER network is not restricted to a perinuclear localization and extends towards the apical pole of airway epithelia, where ER tubules have been described to be in close apposition with the mucin granules in goblet cells from HBE.28 As IRE1β is an ER-resident protein, it is not surprising to see its staining throughout the goblet cell theca where post-Golgi mucin granules are stored (Figure 2a). Quantification of the IRE1β immunostain confirmed that its expression is upregulated in CF as compared with normal HBE (Figure 2b). Moreover, in agreement with the CF findings, freshly isolated HBE from native asthmatic airways exhibited increased levels of IRE1β mRNA vs. normal HBE (Figure 2c). Together, these findings suggest that IRE1β expression is upregulated in airway epithelia from diseases characterized by increased goblet cell expression.
Inositol-requiring enzyme-1 (IRE1)β expression is upregulated in airway diseases characterized by increased expression of goblet/mucous cells. (a) Representative images of IRE1β immunostain in native non-inflamed normal (left panels) and in native inflamed cystic fibrosis (CF) human bronchial epithelia (HBE; right panels). Magnification: × 40 (top panels) and × 100 (lower panels). (b) Quantification of IRE1β expression in native non-inflamed normal and native inflamed CF HBE. Data are representative of three normal and three CF lungs. (c) IRE1β mRNA expression is upregulated in freshly isolated bronchial epithelia from human asthmatic lungs. n=4 normal and 4 asthmatic lungs. *P<0.05, CF vs. normal or asthma vs. normal.
Ire1β−/− mice exhibit decreased mucous cell content and goblet cell number in the nasopharynx
To further address whether the expression of IRE1β correlates with epithelial mucous cell content in vivo, nasopharynx tissues were obtained from untreated Ire1β+/− and Ire1β−/− mice, and subjected to Alcian Blue–Periodic Acid Schiff (AB–PAS) staining. IRE1β is expressed in the nasopharynx (Figure 1a,b), and murine nasopharynx epithelia exhibit a high “baseline” expression of mucous cells, in contrast with epithelia from lower murine airways, which need to be challenged to exhibit significant numbers of mucous cells. The AB–PAS stain was significantly decreased in nasopharynx epithelia from Ire1β−/− vs. Ire1β+/− mice (Figure 3a–c). These findings correlated with a decreased goblet cell number in nasopharynx epithelia from mice lacking IRE1β (Figure 3d), suggesting a role for IRE1β in the maintenance of normal mucus production in vivo.
Inositol-requiring enzyme-1 (IRE1)β expression is associated with the content of mucous cells in murine nasopharynx epithelia. (a,b) Nasopharynx sections from Ire1β+/− mice (a) and Ire1β−/− mice (b) stained with Alcian Blue–Periodic Acid Schiff (AB–PAS) for visualization of goblet cells. (c) Compiled data from a and b illustrating a decreased AB–PAS staining in nasopharynx epithelia from Ire1β−/− mice. (d) Quantification of goblet cells from the nasopharynx epithelia of Ire1β+/− and Ire1β−/− mice. n=4 animals per group. *P<0.05, IRE1β−/− vs. IRE1β+/−.
In murine airways, Clara cells make low levels of mucins without storing mucin granules, and then can transdifferentiate into mucus producing cells in response to ovalbumin (OVA) or interleukin-13 (IL-13). To further investigate the features of IRE1β expression, murine airway sections were co-immunostained for IRE1β and Clara cell secretory protein (CC10), a marker of Clara (and mucous) cells, with and without OVA challenge to induce the differentiation of Clara cells into mucous cells.29 Figure 4a depicts the OVA sensitization and challenge protocol utilized, and when the tissue was procured in these studies. In sections from saline-challenged Ire1β+/− mice, IRE1β was localized to airway epithelial cells and the CC10 staining was faint (Figure 4b, top left panel). In contrast, in OVA-challenged mice, CC10 expression was upregulated and, importantly, there was a significant colocalization of IRE1β and CC10 (Figure 4b, top right panel). Overall, the distribution of IRE1β appeared polarized towards the apical domain of positive cells, in agreement with previous studies reporting the apical polarization of the ER in airway epithelia.3, 4, 30 IRE1β was not expressed in airway sections from Ire1β−/− mice challenged with saline or OVA (Figure 4b, bottom panels), confirming the absence of the protein in these animals and the specificity of the IRE1β antibody. Of note, however, OVA-induced airway epithelial expression of CC10 was blunted in Ire1β−/− mice (Figure 4b, bottom right panel).
Inositol-requiring enzyme-1 (IRE1)β is expressed in Clara cells and ovalbumin (OVA)-induced mucous cells, but not in ciliated cells. (a) Protocol for OVA sensitization and challenge of murine airways. Sensitization is induced on days 0 and 14 via intraperitoneal injection of 0.02% OVA, followed by airway challenge with aerosolized 1% OVA on days 24–26. Lungs are collected on day 31. Saline is used as a control for OVA. (b; top panels): Ire1β (green) and Clara cell secretory protein (CC10; red) immunostains in large airways from Ire1β+/− mice challenged with saline (left) or OVA (right). Ire1β colocalizes with CC10 in airway epithelia from Ire1β+/− mice challenged with OVA. Colocalization of Ire1β and CC10 is shown in yellow. (b; bottom panels): Ire1β is absent in airway epithelia from Ire1β−/− mice, and CC10 expression is blunted in airway epithelia from saline- (left) or OVA-challenged (right) Ire1β−/− mice. n=3 animals per group. (c; top left and right panels): Ire1β (red) and cilia (green) immunostains in airways from saline-challenged Ire1β+/− mice. Ire1β is expressed in Clara cells, but not in ciliated cells of murine airway epithelia. (c; bottom panels): Ire1β is absent in ciliated cells (left), but present in mucous cells (right) from OVA-challenged airways of IRE1β+/− mice. Right bottom panel in c illustrates only mucous cells. n=3 animals per group.
To address whether IRE1β is also expressed in ciliated cells, the same sections were immunostained for IRE1β and ciliated cell-specific tubulin.31 IRE1β staining was not found in tubulin-positive ciliated cells, regardless of whether the airways were challenged with saline (Figure 4c, top panels) or OVA (Figure 4c, bottom panels).
Evidence for a functional role of IRE1β in airway epithelial mucin production in vivo
As IRE1β is functionally relevant in intestinal inflammation,17 we hypothesized that it would also be functionally relevant during an airway inflammatory challenge. We specifically hypothesized a role for IRE1β in OVA-induced allergic airway inflammation. In the absence of OVA, the airway epithelial AB–PAS staining was negligible in both WT and Ire1β−/− mice (Figure 5a). However, the OVA-induced increase in AB–PAS staining observed in WT mice was attenuated in Ire1β−/− mice (Figure 5a,b). Similarly, Muc5b levels, as analyzed by immunohistochemistry, were upregulated by OVA in WT mice and this response was significantly blunted in Ire1β−/− mice (Figure 5c,d). OVA increased AB–PAS staining and Muc5b expression in Ire1β+/− mice were similar to the levels observed in WT mice (data not shown). These findings suggest that IRE1β is required for OVA-triggered upregulation of mucin production.
Ovalbumin (OVA)-induced mucin production is blunted in Ire1β−/− mice. (a) Alcian Blue–Periodic Acid Schiff (AB–PAS) staining of mucous cells in wild-type (WT) and Ire1β−/− mice exposed to saline (−OVA) or OVA. (b) Quantification of mucous cell expression as a percentage of AB–PAS stain/surface area of airway epithelia. Data were derived from large airways. n=3 animals per group. *P<0.05, Ire1β−/− vs. WT mice exposed to OVA. (c) Muc5b staining in airway epithelia from WT and Ire1β−/− mice exposed to saline (−OVA) or OVA. (d) Quantification of Muc5b expression in large airways as a percentage of Muc5b stain/surface area of airway epithelia. n=3 animals per group. *P<0.05, Ire1β−/− vs. WT mice exposed to OVA. (e,f) WT and Ire1β−/− mice exhibit a similar OVA-induced inflammatory response (e: OVA-upregulated interleukin (IL)-13 mRNA expression; (f) OVA-induced eosinophilic airway infiltration). (f) Insets illustrate the absence (left panels) or presence (right panels) of eosinophils infiltrating the airways.
To address whether the decreased OVA-induced mucin production in IRE1β−/− mice resulted from a decreased OVA-elicited inflammatory response, we evaluated OVA increased IL-13 mRNA levels in whole lungs from WT and Ire1β−/− mice. In saline-challenged animals, IL-13 mRNA levels were low; however, OVA upregulated IL-13 mRNA levels, and this response was similar in WT vs. Ire1β−/− mice (Figure 5e). Assessment of the eosinophilic infiltration characteristic of OVA-induced inflammation further illustrated that the inflammatory response is not impaired in Ire1β−/− mice (Figure 5f). These findings suggest that the blunted OVA-induced mucin production in Ire1β−/− mice does not result from an underlying reduction in the inflammatory response.
ER stress genes in saline and OVA-challenged WT vs. IRE1β−/− mice
We next evaluated the levels of ER stress genes, which are markers of activation of the ATF6 and PERK branches of the UPR. We assessed total (unspliced) XBP-1 and immunoglobulin binding protein (BIP; also known as GRP78), whose levels are upregulated resulting from ATF6 activation, and ATF4 and CHOP, which are upregulated as a consequence of PERK activation, in saline and OVA-challenged mice. No significant differences were found between the mRNA levels of these ER stress genes in tracheas from WT vs. Ire1β−/− mice challenged with saline or OVA (Figure 6a). The only gene that trended to be different between OVA-challenged WT vs. Ire1β−/− mice was Bip. Nonetheless, as an index of ER stress, OVA increased XBP-1 mRNA splicing in WT mice, whereas this response was decreased in Ire1β−/− mice (Figure 6b). Analyses of the same tracheal samples for mucous cell genes (e.g., ClCa3, Muc5ac, and Agr2), revealed that although OVA exposure upregulated these genes in WT mice, the response was blunted in Ire1β−/− mice (Figure 6c). Together, these data lead to the notion that activation of IRE1β during OVA-induced mucous cell transdifferentiation promotes XBP-1 mRNA splicing associated with the upregulation of mucous cell genes.
Endoplasmic reticulum (ER) stress and mucous cell gene expression in saline- and OVA-challenged wild-type (WT) vs. IRE1β−/− mice. (a) mRNA expression levels of total X-box binding protein-1 (XBP-1), immunoglobulin binding protein (BIP)/GRP78, activating transcription factor 4 (ATF4), and CHOP in tracheas from saline and OVA-challenged WT vs. Ire1β−/− mice. (b) OVA increases XBP-1 mRNA splicing in WT mice, and this response is blunted in Ire1β−/− mice. *P<0.05, Ire1β−/− vs. WT mice exposed to OVA. (c) OVA induces upregulation of mucous cell genes in WT mice, and this response is blunted in Ire1β−/− mice. *P<0.05, Ire1β−/− vs. WT mice exposed to OVA. KO: Ire1β−/− (knockout) mice.
IRE1β does not regulate BIP, ATF4, or CHOP genes, but is required to maintain normal transcriptional levels of mucin genes and genes linked to mucin/mucus production in vitro
We further evaluated the functional role of IRE1β using short hairpin RNA (shRNA) knockdown in polarized cultures of Calu-3 cells, an airway epithelial cell line that expresses mucous cells and produces mucins,32 treated with IL-13 to induce mucus cell transdifferentiation. Expression of pSIREN IRE1β shRNA decreased the baseline mRNA levels of IRE1β by ∼50% (Figure 7a, t=0). Congruent with the OVA studies in mice, endogenous IRE1β mRNA levels increased in a time-dependent manner after serosal application of IL-13 in control vector-expressing cells, and this response was blunted in cells expressing the IRE1β shRNA (Figure 7a). Albeit to a lower extent, IL-13 upregulated the IRE1α mRNA in control cells, but this response was not blunted in IRE1β shRNA-expressing cells (Figure 7a), illustrating the specific effect of the IRE1β shRNA on IL-13 upregulated IRE1β mRNA levels.
Inositol-requiring enzyme 1 (IRE1)β gene expression is associated with the expression of mucous cell genes in Calu-3 cell cultures. Time courses for 50 ng ml−1 serosal interleukin (IL)-13-modulated IRE1β and IRE1α gene expression (a), immunoglobulin binding protein (BIP)/GRP78, activating transcription factor 4 (ATF4) and CHOP, (b) and MUC5B and MUC5AC (c) in polarized cultures of Calu-3 cells stably expressing a control pSIREN vector (black bars) or a pSIREN vector containing a IRE1β shRNA (white bars). Data depict reverse transcriptase-PCR (RT-PCR) analyses. Data are from four experiments. *P<0.05, pSIREN IRE1β shRNA-expressing cells vs. pSIREN control-expressing cells. (d; left): Correlation coefficient analysis between IRE1β gene expression and SAM pointed domain containing ETS transcription factor (SPDEF; P-value for correlation is shown on top of figure). (d; right): Time courses for 50 ng ml−1 serosal IL-13-modulated SPDEF gene expression in polarized cultures of Calu-3 cells stably expressing a control pSIREN vector (black bars) or a pSIREN vector containing a IRE1β shRNA (white bars). Data depict RT-PCR analyses. (e; left): Correlation coefficient analysis between IRE1β gene expression and anterior gradient homolog 2 (AGR2) gene expression (P-value for correlation is shown on top of figure.). (e; right): Western blot (representative from three experiments) depicting the time course for 50 ng ml−1 serosal IL-13-induced AGR2 protein expression from whole-cell lysates from polarized cultures of Calu-3 cells stably expressing the control vector (“−” on top of figure) or IRE1β shRNA (“+” on top of figure). β-actin is used as a loading control.
Importantly, similar to the in vivo murine data, neither BIP, ATF4, nor CHOP mRNA levels were different in vehicle- or IL-13-treated Calu-3 cells expressing the control vector vs. the IRE1β shRNA (Figure 7b). Treatment with IL-13 for 72 h upregulated the mRNA levels of CHOP, but this response was not blunted in cells expressing the IRE1β shRNA (Figure 7b).
On the other hand, although IL-13 upregulated both MUC5B and MUC5AC mRNA levels in cultures stably expressing the control vector, this effect was attenuated in cultures stably expressing the IRE1β shRNA (Figure 7c). Additional analyses using all available time points from the IL-13 studies demonstrated significant positive correlations between the mRNA levels for IRE1β and the mRNA levels for SPEDF (Figure 7d, left panel). Notably, further evaluation of the role of IRE1β on IL-13-induced SPDEF gene expression revealed that, although IL-13 upregulated the mRNA levels of SPDEF, this response was not blunted by the IRE1β shRNA (Figure 7d, right panel). In contrast, our studies revealed the existence of a significant positive correlation between the mRNA levels for IRE1β and AGR2 (Figure 7e, left panel), which reflected a regulation of AGR2 mRNA levels by IRE1β, based on evaluation of the AGR2 protein levels; although IL-13 increased in a time-dependent manner the AGR2 protein levels in control vector-expressing cells, this response was blunted in cells expressing the IRE1β shRNA (Figure 7e, right panel). These findings suggest that IL-13-upregulated IRE1β mRNA expression correlates with the upregulation of the mRNAs from both SPDEF and AGR2, illustrating the participation of these genes in the mucous cell transdifferentiation process. However, IRE1β appears to be only functionally relevant for IL-13-promoted AGR2 expression in Calu-3 cells.
On the basis of these findings with the Calu-3 cells illustrating that ∼50% knockdown of IRE1β levels is sufficient to decrease mucin production (Figure 7), it could be argued that haploinsufficiency may influence mucin production in Ire1β+/− mice; however, the data from Figure 3 illustrate that significant phenotypical differences were found between Ire1β+/− vs. Ire1β−/− mice, in accord with the Calu-3 cell data.
Reduction of IRE1β decreases cellular mucin content and mucin secretion
Reduction in mucin gene mRNA levels in IRE1β shRNA-expressing cultures resulted in a parallel reduction in mucin protein, indicated by a reduction in both basal and IL-13-induced MUC5AC and MUC5B in whole-cell lysates evaluated by western blots (Figure 8). Basal protein levels were decreased for both mucins in IRE1β shRNA-expressing cells (Figure 8a–d). In addition, IL-13-upregulated MUC5AC (Figure 8e, left) and MUC5B (Figure 8e, right) protein production was blunted in cells expressing IRE1β shRNA, which was especially notable at 72 h after addition of IL-13. Immunocytochemical detection of MUC5AC from Calu-3 cell cultures paralleled these findings (Figure 8f). Importantly, the decreased basal cellular mucin content in cells with knocked-down levels of IRE1β was coupled to a decreased basal secretion of MUC5AC protein (Figure 8g,h).
Reduction of inositol-requiring enzyme 1 (IRE1)β decreases basal and interleukin (IL)-13-stimulated cellular mucin content and mucin secretion. (a) Western blot from a 1% agarose gel depicting the staining for basal levels of MUC5AC protein expression from whole-cell lysates from polarized cultures of Calu-3 cell expressing a pSIREN control vector or a pSIREN vector containing a IRE1β shRNA. Data are representative of three individual experiments. (b) Compiled data from the MUC5AC signals from a. (c) Western blot from a 1% agarose gel depicting the staining for basal levels of MUC5B protein expression from whole-cell lysates from polarized Calu-3 cell cultures expressing a pSIREN control vector or a pSIREN vector containing an IRE1β shRNA. Data are representative of three individual experiments. (d) Compiled data from the MUC5B signals from c. (e) Western blots from 1% agarose gels depicting the time course for 50 ng ml−1 serosal IL-13-induced MUC5AC (left) and MUC5B (right) protein expression from whole-cell lysates from polarized Calu-3 cell cultures expressing a pSIREN control vector or a pSIREN vector containing an IRE1β shRNA. (f) Immunocytochemical detection of MUC5AC from Calu-3 cell cultures expressing a pSIREN control vector (left) or a pSIREN vector containing a IRE1β shRNA (right). Bar (right panel) = 20 μm. (g) Slot blots for basal MUC5AC secreted protein in Calu-3 cells expressing a pSIREN vector containing a IRE1β shRNA vs. Calu-3 cells expressing a pSIREN control vector. (h) Compiled data from the MUC5AC signals depicted in g. Data are representative of three to four experiments. *P<0.05, pSIREN IRE1β shRNA-expressing cells vs. pSIREN control-expressing cells.
We evaluated the impact of knocking down IRE1α on mucin production in Calu-3 cultures stably expressing an IRE1α shRNA. Cultures expressing the IRE1α shRNA exhibited ∼75% decrease in IRE1α mRNA levels (Figure 9a). The mRNA levels of IRE1β were not blunted but, rather, increased in cultures expressing the IRE1α shRNA, and this response was similar for both the mRNA levels of MUC5AC and MUC5B (Figure 9a). In addition, contrary to cells expressing the IRE1β shRNA, Calu-3 cultures expressing the IRE1α shRNA did not have a decreased cellular content of MUC5AC or a decreased basal secretion of MUC5AC protein (Figure 9b–d).
Inositol-requiring enzyme 1 (IRE1)α knockdown does not affect mucin production in Calu-3 cells. (a) mRNA levels of IRE1α, IRE1β, MUC5AC, and MUC5B in Calu-3 airway epithelial cultures expressing a control vector (black bars) or a vector containing an IRE1α shRNA (white bars). (b) Immunocytochemical detection of MUC5AC from Calu-3 cell cultures expressing a control vector (left) or a vector containing an IRE1α shRNA (right). Bar (left panel) = 20 μm. (c) Slot blot for basal MUC5AC secreted protein in Calu-3 cells expressing a vector containing an IRE1β shRNA vs. Calu-3 cells expressing a control vector. (d) Compiled data from the MUC5AC signals depicted in c. Data are representative of three experiments.
Overexpression of IRE1β increases cellular mucin content and mucin secretion
Studies in Ire1β−/− mice and IRE1β shRNA studies in Calu-3 cells revealed that normal IRE1β levels are required to maintain normal production of mucins and mucin-related genes. On the basis of these findings, we predicted that overexpression of IRE1β would result in augmentation of mucous cell responses. Overexpression of IRE1β via a retroviral expression vector (pQCXIN) in Calu-3 cells resulted in the upregulation of the IRE1β mRNA levels (Figure 10a). In addition, we found that MUC5AC protein production was increased in cells overexpressing IRE1β as compared with cells containing a control pQCXIN vector (Figure 10b). Moreover, the secreted basal levels of MUC5AC protein were augmented in cells overexpressing IRE1β (Figure 10c,d).
Overexpression of inositol-requiring enzyme 1 (IRE1)β increases cellular mucin content and basal mucin secretion. (a) IRE1β mRNA levels in Calu-3 cell cultures expressing a control pQCXIN vector or a pQCXIN vector containing IRE1β. (b) Immunocytochemical detection of MUC5AC from Calu-3 cell cultures expressing a control pQCXIN vector (left) or a pQCXIN vector containing IRE1β (right). Bar (left panel) = 20 μm. (c) Slot blots for basal MUC5AC secreted protein in Calu-3 cells expressing a pQCXIN vector containing IRE1β vs. Calu-3 cells expressing a control pQCXIN vector. (d) Compiled data from the MUC5AC signals depicted in c. *P<0.05, Calu-3 cells expressing a pQCXIN vector containing IRE1β vs. Calu-3 cells expressing a control pQCXIN vector. Data are representative of three to four individual experiments.
All together, the data from Figures 8 and 10 indicate that IRE1β is required for airway epithelial mucin production in vitro, in agreement with its functional role in the regulation of mucin production in vivo. Our findings further suggest that this function is specific for IRE1β vs. IRE1α, as mucin production was not blocked in Calu-3 cultures expressing the IRE1α shRNA (Figure 9).
IRE1β-dependent mucin production is mediated by activation of XBP-1-induced transcription of AGR2
Since activation of XBP-1 mRNA splicing has a fundamental role in secretory responses in many cells,33, 34, 35, 36, 37 including airway epithelia,3, 4, 5, 38 we evaluated whether IL-13-induced mucin production coupled to XBP-1 mRNA splicing in Calu-3 cultures. The same samples utilized in the studies described in Figure 7a were used in these studies. IL-13 promoted XBP-1 mRNA splicing in a time-dependent manner in Calu-3 cells (Figure 11a,b). Significantly, this response was mediated by IRE1β, as indicated by a reduction in IL-13-triggered XBP-1 mRNA splicing in cells expressing the IRE1β shRNA, in agreement with the data from IRE1β−/− mice challenged with OVA (Figure 6b).
Inositol-requiring enzyme 1 (IRE1)β-dependent mucin production is mediated by activation of X-box binding protein-1 (XBP-1) coupled to upregulation of anterior gradient homolog 2 (AGR2). (a) Representative Southern blot illustrating that interleukin (IL)-13 triggers XBP-1 mRNA splicing in Calu-3 cell cultures expressing a pSIREN control vector, and this function is blunted in cultures expressing a pSIREN vector containing a IRE1β shRNA. (b) Compilation of the XBP-1 mRNA splicing data expressed as a percentage of XBP-1 mRNA splicing from t=0 from control cultures. Data were derived from the same cDNA samples used in Figure 7 to assess the mRNA levels of IRE1β. (c): AGR2 mRNA expression in Calu-3 cells expressing a control pQCXIN vector, a pQCXIN vector containing spliced XBP-1, or a pQCXIN vector containing a dominant-negative XBP-1 (DN-XBP-1). *P<0.05, spliced XBP-1-expressing cells vs. control cells; # P<0.05, DN-XBP-1-expressing cells vs. control cells. (d) Immunocytochemical detection of MUC5AC from Calu-3 cell cultures expressing a control pQCXIN vector (left), a pQCXIN vector containing spliced XBP-1 (center), or a pQCXIN vector containing a DN-XBP-1 (right). Bar (left figure) = 20 μm. (e) Slot blots illustrating the basal levels of MUC5AC secreted protein in Calu-3 cells expressing a control pQCXIN vector, a pQCXIN vector containing spliced XBP-1, and a pQCXIN vector containing a DN-XBP-1. (f) Compiled data from the MUC5AC signals depicted in e. *P<0.05, spliced XBP-1- or DN-XBP-1-expressing cells vs. control cells; #P<0.05, spliced XBP-1- vs. DN-XBP-1-expressing cells. Data are from three experiments.
Because these findings further suggested that IRE1β-dependent mucin production is mediated by activation of XBP-1, Calu-3 cultures stably expressing the active, spliced form of XBP-1, or a dominant-negative XBP-1 (DN-XBP-1)5, 39 were generated to directly test the functional role of XBP-1 in mucin production. As our studies revealed that IRE1β regulates AGR2 expression both in vivo (Figure 6c) and in vitro (Figure 7e), and AGR2 is functionally important for intestinal and airway epithelial mucin production,22, 23 we evaluated the AGR2 mRNA levels in the three cell lines. As compared with control cultures, cultures expressing the spliced XBP-1 exhibited an upregulation of AGR2 mRNA, whereas expression of the DN-XBP-1 decreased by ∼50% the AGR2 mRNA levels (Figure 11c). Similar findings were obtained for MUC5B mRNA levels (data not shown). These responses mirrored the results for MUC5AC protein—as compared with control cultures, the expression of spliced XBP-1 upregulated the production of MUC5AC, whereas the expression of the DN-XBP-1 decreased the MUC5AC content (Figure 11d). The changes in intracellular levels of MUC5AC in spliced XBP-1- or DN-XBP-1-expressing cultures corresponded to similar changes in the basal secreted levels of this mucin (Figure 11e,f).
These novel findings suggest that IRE1β-dependent airway epithelial mucin production is mediated, at least in part, by XBP-1 activation-induced upregulation of AGR2 expression, which in turn is essential for airway epithelial mucin production.23
Discussion
Similar to what has been discovered for other professional secretory cells (e.g., plasma cells), it is expected that the development of airway mucous cells, which are specialized secretory cells that produce and secrete mucins, would require activation of UPR pathways to maintain their secretory capacity. Although activation of UPR pathways has been implicated in the pathogenesis of several diseases,11 only a few studies have addressed the role of the UPR in airway inflammation.38, 40, 41, 42 The present study has uncovered a novel and unsuspected role for IRE1β, an isoform of IRE1α and a mediator of the mammalian UPR response, in the regulation of airway mucin production. Our study also indicates that this function is specific for IRE1β vs. IRE1α.
Our findings demonstrate IRE1β expression in non-ciliated cells lining the conducting airways, including goblet/mucous cells in humans, and Clara and mucous cells in mice (Figures 2 and 4). IRE1β couples to the regulation of mucin production and secretion (Figures 3, 5, 6, 7 and 8 and 10), and is upregulated by a key inflammatory mediator, IL-13, which upregulates mucin production in airways (Figures 7 and 8). Importantly, IRE1β is required for the upregulation of mucin production after OVA- or IL-13-induced mucous cell transdifferentiation (Figures 5, 6, 7 and 8). In fact, the upregulation of IRE1β mRNA levels in freshly isolated airway epithelia from asthmatic subjects (Figure 2) suggests a role for IRE1β in the pathogenesis of asthma. Moreover, the localization of IRE1β in human CF airway goblet cells (Figure 2), where production of goblet cells is thought to be driven by infection, suggests that IRE1β is functional during a variety of diseases leading to airway goblet cell transdifferentiation.
Compared with IRE1α, IRE1β has been less studied and its mechanism of action is less clear. Both genes show the same overall structure, but they are only 39% identical at the amino acid level overall, although the identity is higher in the functionally relevant kinase (80%) and RNase (61%) domains.43 IRE1β was reported to be competent to cleave XBP-1 mRNA,13 but it apparently does so less efficiently than IRE1α in HeLa cells.44 Overexpression of IRE1β in HeLa cells induces 28S rRNA cleavage more efficiently than IRE1α,44 and it was suggested that IRE1β acts through 28S ribosomal RNA cleavage to induce translational repression during ER stress responses. This translational repression was later shown to be specific to ER-associated (as opposed to cytoplasmic) translation.45 These studies were conducted in non-mucous-producing cells, and they may not reflect a completely accurate picture of IRE1β function.
No previous study had linked IRE1β function to regulation of airway mucin production, but available databases led us to consider this possibility, and we demonstrated the utility of these data sets for establishing our hypothesis and to query gene function. UNIGENE expression data predicted high levels of IRE1β in conducting airways, and this was confirmed, despite previous literature referencing IRE1β expression specifically to gut epithelium.17 In addition, the Gene Expression Tool Celsius (UGET; http://genome.ucla.edu/projects/UGET) revealed high correlations between the IRE1β gene expression and the expression of genes linked to mucin/mucus production, such as AGR2, CLCA1/3, SPDEF, and mucin glycosylating enzymes (Supplementary Material online and Supplementary Table 1 online). Lack of association of IRE1α expression with these same genes led us to hypothesize a mucous cell-specific role for IRE1β. Indeed, our findings demonstrate that absence of IRE1β or IRE1β knockdown reduced the levels of mucous cell-specific genes (Figures 6 and 7), consistent with the UGET correlations, and this effect was dissociated from alterations in IRE1α expression (Figures 7 and 9).
We evaluated the relevance of IRE1β-regulated ER stress genes in two models functionally important for mucin production: (1) tracheas from OVA-challenged WT vs. IRE1β−/− mice and (2) IL-13-exposed control and IRE1β shRNA-expressing Calu-3 cells. These studies revealed the following: First, although the mRNA levels of ER stress genes were similar in tracheas from WT vs. IRE1β−/− mice, OVA increased XBP-1 mRNA splicing in WT mice and this response was decreased in IRE1β−/− mice (Figure 6). The blunted OVA-induced XBP-1 mRNA splicing was associated with decreases in OVA-induced mucous cell genes (e.g., ClCa3, Muc5ac, and Agr2) in tracheas from IRE1β−/− mice (Figure 6). Second, similar to the data from the IRE1β−/− mice, Calu-3 cells with IRE1β knockdown exhibit decreased IL-13-induced XBP-1 mRNA splicing (Figure 11), and decreased mRNA and protein values of mucins and AGR2 (Figures 7 and 8). Third, similar to the murine data, neither BIP, ATF4, nor CHOP mRNA levels were different in vehicle or IL-13-treated control vs. IRE1β shRNA-expressing Calu-3 cells (although treatment with IL-13 for 72 h upregulated CHOP mRNA, this response was not blunted in cells expressing the IRE1β shRNA (Figure 7)).
Reduction of IRE1β also led to decreased mucin protein expression and secretion (Figure 8), whereas overexpression of IRE1β increased mucin protein production and secretion (Figure 10). The latter findings do not support the hypothesis that IRE1β’s role is limited to the destruction of mRNAs encoding secreted proteins, as previously described.45 Although the present study has not directly addressed protein translation, our data suggest that IRE1β expression is necessary to maintain the secretory capacity of mucin-producing cells, under both basal and stimulated conditions (Figures 3, 5, 6, 7 and 8).
Considering the similar findings from the mouse and Calu-3 cell studies, our data suggest that the downregulation of mucin production resulting from loss or decrease of IRE1β is independent of ER stress-induced activation of ATF6 or PERK. Consistent with our data, Agr2−/− mice exhibit only a very modest ER stress in intestinal epithelia.22 These observations lead to the view that the decreased mucin production resulting from lack of IRE1β is not mediated by a robust, canonical ER stress response. Instead, our data suggest that some sort of failure in the maturation of goblet cells occurs in airway epithelia lacking IRE1β. In agreement with this notion, lack of IRE1β in murine mucous cells results in decreased airway epithelial goblet cell number (Figure 3). The finding that mucin-producing cells are not completely absent in Ire1β−/− mice (Figures 3 and 5) is in agreement with the previous observations that mucous cells are not entirely missing in Spdef−/− mice,20 and airway epithelial Muc5ac and Muc5b production is attenuated (but not abolished) in allergen-sensitized and challenged Agr2−/− mice.23
The reduction in mucous cell-associated genes/proteins that are not secreted, such as AGR2, when IRE1β is absent or reduced (Figures 6 and 7), suggests that IRE1β function does have a global effect on the mucous cell transdifferentiating response, perhaps by increasing the ability to maintain the mucous cell phenotype under conditions when a large secretory capacity is demanded. Our findings lead to the view that the airway epithelium fails to maintain a fully differentiated mucous cell phenotype in the absence of IRE1β (an allostatic feedback due to failure to maintain an adequate secretory apparatus). More interestingly, our findings also suggest a role for IRE1β (but not for IRE1α) signaling in maintaining mucin gene expression. We further speculate that, unlike IRE1α, IRE1β has evolved a function to recognize specific motifs in mucins, e.g, glycosylation domains, which could be the trigger for its activation during airway inflammation associated with mucin overproduction. These features of IRE1β make it attractive as a therapeutic target for reducing, but not completely eliminating, mucous cell responses, which are likely necessary for maintaining a certain level of mucus ciliary clearance during pulmonary disease progression.
Although IRE1α deficiency leads to embryonic lethality due to placental deficiency,15 Ire1β−/− mice are viable and it was only after challenge with DSS that a colitis phenotype emerged.17 On the basis of our findings that IRE1β is involved in mucin production, we speculate that the increased susceptibility of Ire1β−/− mice to DSS17 could be due to reduction of intestinal Muc2 production. This hypothesis is congruent with studies that detected missense mutations in Muc2 in mice that develop a colitis-like phenotype after a random mutagenesis screen.18 These mutant mice showed aberrant Muc2 biosynthesis, decreased mucin content in mucous cells, a diminished mucus barrier, and an increased susceptibility to DSS-induced colitis.18 Thus, decreased mucin production is likely a common pathogenic mechanism in both Muc2 mutant and Ire1β−/− mouse models.
Our findings indicate that the mechanism of OVA- or IL-13-induced mucin overproduction in airways is mediated, at least in part, by IRE1β-dependent activation of XBP-1. Indeed, we show that the intracellular mucin content and the basal levels of mucin secretion in Calu-3 cells are upregulated by expression of the spliced XBP-1 and blunted by expression of a DN-XBP-1 (Figure 11). In agreement with our findings, transgenic mice lacking XBP-1 specifically in intestinal epithelial cells exhibit a decreased number of goblet cells per villus, smaller cytoplasmic mucin granules in goblet cells, and a contracted ER.46 These latter observations are also compatible with our previous findings that XBP-1 mRNA splicing triggers expansion of the ER in airway epithelia.5 Notably, the intestinal epithelia Xbp1−/− mouse is also more susceptible to DSS-induced colitis,46 in agreement with Ire1β−/− and the Muc2 mutant mouse models.17, 18
Moreover, our data suggest that AGR2 is functionally implicated in IRE1β-dependent XBP-1-mediated mucin production for the following reasons. First, IRE1β regulates AGR2 expression resulting from OVA challenges in vivo or IL-13 treatment in vitro (Figures 6 and 7). Second, a role for AGR2 in IRE1β/XBP-1-dependent mucin production is further suggested by our findings that Calu-3 cells expressing the spliced (active) XBP-1 have an upregulation of AGR2 mRNA coupled to increased mucin production, whereas cells expressing a DN-XBP-1 exhibit decreased AGR2 mRNA levels and decreased mucin content (Figure 11). Third, recent studies have shown that AGR2 is functionally important for the regulation of airway epithelial mucin production.23 These observations lead to the proposal that activation of IRE1β during mucous cell transdifferentiation promotes XBP-1 mRNA splicing-dependent transcription of AGR2 which, in turn, is responsible for, at least in part, the regulation of mucin production in airway epithelia. On the basis of the similar functional roles of IRE1β, XBP-1, and AGR2 in airways and gut physiology, and pathogenesis related to mucin production, this proposed model provides a fundamental mechanism involving the UPR relevant to the innate defenses of both the airways and the gut.
Although not the focus of the present study, it is also possible that a mechanism involving a regulated IRE1β-dependent decay (RIDD) of mRNA, consistent with previous reports of IRE1β-mediated mRNA degradation,44, 47 may contribute to the health of differentiated mucin-producing cells. This alternative model would suggest that the role of IRE1β in maintaining mucin production is carried out indirectly via its effect on cellular dynamics. In fact, the two mechanisms, e.g., IRE1β/XBP-1-dependent AGR2-mediated mucin production and IRE1β-dependent RIDD can co-exist. Further studies will be necessary to address the possible function of IRE1β-dependent RIDD in airway epithelial mucous cell transdifferentiation.
As chronic airway diseases characterized by hypersecretion of mucins and mucus obstruction, such as asthma, CF, and chronic obstructive pulmonary disease, constitute a major burden to public health, a considerable amount of research has been devoted to identify protein targets that contribute to airway mucin overproduction. Utilizing a multidisciplinary approach and in vivo and in vitro models relevant to airway mucin overproduction, our studies demonstrated that IRE1β is specifically localized in airway mucous cells, where it is necessary to maintain mucin production. These novel findings offer the initial proof of concept that IRE1β is a potential therapeutic target for airway inflammatory diseases characterized by mucus overproduction. The recent development of IRE1α-specific endoribonuclease inhibitors,48 coupled with the lack of complete homology between IRE1α and IRE1β in the RNase domain,43 suggests that drugs specifically targeted against IRE1β activity can be found and used as anti-mucus therapeutic agents.
Methods
Mice. Six- to eight-week-old WT, Ire1β+/−, and Ire1β−/− mice were used in this study. The generation of Ire1β−/− mice has been previously described.17 All animal protocols were approved by The Institutional Animal Care and Use Committee of The University of North Carolina at Chapel Hill.
HBE cell culture and freshly excised tissue. Tissues and cells were obtained under the auspices of protocols approved by the Institutional Committee on the Protection of the Rights of Human Subjects and provided by the UNC CF Center Tissue Core. Tissues from normal, CF, and asthmatic lungs were obtained from main stem or lobar bronchi as previously described,3, 4, 5 and either immersion-fixed at 4 °C in Omnifix II (FR Chemical, Mount Vernon, NY) and embedded in paraffin or used for cell isolation and culturing as reported.3, 4, 5 HBE cultures were subject to mRNA analyses of IRE1β, MUC5B, AGR2, and SPDEF. Alternatively, freshly isolated asthmatic HBE were subjected to mRNA analyses of IRE1β.
Calu-3 cultures. Calu-3 cells were obtained from ATCC (Manassas, VA). For studies with knockdown of IRE1β, pSIREN IRE1β shRNA and pSIREN control retroviral vectors were used to infect and generate stable cell lines using standard methods. The siRNA sequence 5′-CGAACACAGTATACGGTCA-3′ (Thermo Scientific/Dharmacon, Lafayette, CO) was cloned into the Knockout RNAi-Ready pSIREN vector (Clontech, Mountain View, CA) to generate shRNA to IRE1β. Cells were grown on Transwell T-Clears (Corning, Tewksbury, MA) and 21-day polarized cultures were subjected to time courses for serosal IL-13 (R&D, Minneapolis, MN; 50 ng ml−1)-regulated IRE1β, IRE1α, BIP, ATF4, CHOP, MUC5AC, MUC5B, SPDEF, and AGR2 mRNA levels, which were assessed by standard techniques.
For studies with knockdown of IRE1α, a control pLKO plasmid or a pLKO plasmid containing a shRNA to IRE1α (clone ID TRCN0000000530; Thermo Scientific/Open Biosystems, Lafayette, CO) were obtained from the UNC lenti-shRNA core facility, packaged into lentivirus according to the manufacturer’s protocol, and used to infect and generate stable cell lines using standard methods. Calu-3 cell cultures were grown and studied under the same conditions utilized for the IRE1β shRNA studies.
Real time-PCR of IRE1β and IRE1α in mouse and human tissues. Utilizing standard protocols, cDNA was obtained from mouse and human tissues and reverse transcriptase-PCR was performed. The following primers (obtained from MWG, Huntsville, AL) were used: mouse IRE1β: 5′-CCTGGGTCCTCTACCTGATG-3′ (forward) and 5′-AAGGAAATCTTCCCCACCAC-3′ (reverse); mouse IRE1α: 5′- CAGATCTGCGCAAATTCAGA-3′ (forward) and 5′-CTCCATGGCTTGGTAGGTGT-3′ (reverse); human IRE1β: 5′-CAGAGTCCCTCAAAGCAAGC-3′ (forward) and 5′-AGCTCCAGGGCAATGTAGTG-3′ (reverse); human IRE1α: 5′-CGGCCT TTGCAGATAGTCTC-3′ (forward) and 5′- ACGTCCCCAGATTCACTG-3′ (reverse). For assessment of 18S, QuantumRNA Universal 18S primer set (Ambion, Grand Island, NY) was used. Quantitative reverse transcriptase-PCR was performed according to a published method.49
Assessment of mRNA levels of Xbp-1, Bip, Atf4, Chop, CaCl3, Muc5ac, Agr2 , and Il-13 in mouse tissues. RNA was isolated by standard methods from tracheas or lungs from WT and Ire1β−/− mice 2 days after saline or OVA challenges. The following Taqman Probes (Applied Biosystems, Grand Island, NY) were used to assess the mRNA levels of the following genes: Xbp-1: Mm00457357_m1; Bip: Mm00517691_m1; Atf4: Mm00515324_m1; Chop: Mm00492097_m1; CaCl3: Mm00489959_m1; Agr2: Mm00507853_m1; Il-13: Mm00434204_m1.
Assessment of mRNA levels of IRE1β, IRE1α, BIP, ATF4, CHOP, MUC5AC, MUC5B, SPDEF, AGR2, and 18S in cultures of Calu-3 cells. RNA was isolated by standard methods from Calu-3 cultures subjected to different treatments and time courses. The following Taqman Probes (Applied Biosystems) were used to assess the mRNA levels of the following genes: IRE1β: Hs00414750_m1; IRE1α: Hs00176385_m1; BIP: Hs99999174_m1; CHOP: Hs01090850_m1; MUC5AC: Hs01365616_m1; MUC5B: Hs00861588_m1; SPDEF: Hs01026048_m1; AGR2: Hs00180702_m1; and 18S: Hs99999901_s1. For ATF4, the following primers were utilized: 5′-TCAAACCTCATGGCTTCTCC-3′ (forward) and 5′-AGGTCATCTGGCATGGTTTC-3′ (reverse).
OVA studies. OVA sensitization and airway challenges of WT, Ire1β+/−, and Ire1β−/− mice were performed according to a conventional protocol consisting of intraperitoneal injections (0.02% OVA) on days 0 and 14, followed by 3 consecutive challenges with aerosolized OVA (2%) on days 24–26. Saline was used as a control. Lung sections were obtained on day 31 and stained with AB–PAS or subjected to immunodetection. For RNA samples, the same sensitization protocol was utilized, animals were challenged on days 24 and 25, and samples were collected on day 26.
Immunodetection studies. Immunofluorescence of IRE1β, CC10, and tubulin was performed in murine lung sections, using a primary rabbit anti-IRE1β antibody (1:250 dilution; from D Ron’s), a mouse anti-CC10 antibody (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), and a rat anti-tubulin antibody (1:250 dilution; Chemicon, Billerica, MA), together with secondary antibodies (Jackson Laboratories, West Grove, PA; 1:500 dilution), according to standard procedures. IRE1β, CC10, and tubulin fluorescences were assessed with a laser-scanning confocal microscope.3, 4, 5, 31 Immunocytochemical detection of Muc5b in mouse airways was performed with a rabbit anti-Muc5b antibody50 (1:1,000 dilution) and a goat anti-rabbit peroxidase secondary antibody (Vector Laboratories, Burlingame, CA) according to the manufacturer’s directions. Immunocytochemical detection of MUC5AC in Calu-3 cells was performed according to a published method,32 with cultures grown for 7–10 days.
For immunostaining of IRE1β in normal and CF native human airway epithelia, bronchial samples were immersion-fixed at 4 °C in Omnifix II (FR Chemical) for 24 h and processed into paraffin. De-waxed 5 μm sections were rehydrated, antigen retrieval was performed at 100 °C for 7 min (DAKO, Carpinteria, CA, pH 6), cooled to room temperature, and washed in distilled water. Endogenous peroxidase activity was blocked with 3% H2O2 in distilled water for 20 min followed by three distilled water washes. A blocking solution consisting of 8% tryptone in 150 mM Tris, pH 7.5, 100 mM NaCl, and 0.1% Tween-20 was applied to the sections for 1 h at room temperature, followed by incubation with the rabbit IRE1β antibody (D. Ron’s) or a rabbit non-immune serum (diluted 1:500 in blocking solution) overnight at 4 °C. Sections were washed three times in Tris/NaCl/Tween-20, incubated with a peroxidase-conjugated anti-rabbit antibody (1:500 in blocking solution; Jackson Laboratories) for 1 h at room temperature, washed as described above, followed by incubation with 0.05% diaminobenzidine and 0.003% H2O2 in 0.1 M sodium acetate, pH 6.0, for 5 min. Sections were washed, hematoxylin-counterstained, dehydrated, and mounted with Cytoseal 60 (Thermo Scientific/Anatomical Pathology, Kalamazoo, MI).
Immunodetection was evaluated either by confocal microscopy (Leica, model TCS 4D, Buffalo Grove, IL; PL APO × 63/1.20 mm water lens) or with an inverted microscope with color, and black and white cooled digital cameras (Leica DMIRB), as previously described.3, 4, 5
Quantification of IRE1β immunostain in sections from native normal and CF airways was performed according to a published method,3 where regions of interest were drawn around the airway epithelial layer stained with the IRE1β antibody, and the signal intensity quantified and expressed in arbitrary units.
Quantification of mucous cell expression in murine airways. Regions of interest were drawn around the airway epithelial layer stained with AB–PAS or the Muc5b antibody, a color threshold generated and applied to airway sections. Metamorph software was used to quantify the AB–PAS or Muc5b staining, which was expressed as a percentage of airway epithelial surface. Quantification of goblet cell number was performed according to a published method.51
Western blots. Assessment of MUC5AC and MUC5B total protein was performed utilizing quantitative 1% agarose gels and western blotting as previously reported.27, 52 Gels were submerged in a gel electrophoresis apparatus with Tris acetate-EDTA/SDS buffer, at 80 V for 90 min, vacuum-blotted onto nitrocellulose membranes, and blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE). The commercially available 45M1 mouse antibody (1:5,000 dilution; Thermo Scientific, Lincoln, NE) was used to stain MUC5AC, whereas a rabbit anti-MUC5B antibody (1:5,000 dilution)50 was used to stain MUC5B. The secondary antibodies were a 680 nm goat anti-mouse for MUC5AC and a 800 nm goat anti-rabbit for MUC5B (LI-COR Biosciences).
For detection of AGR2 protein levels, SDS-polyacrylamide gel electrophoresis and western blots were performed, using a primary rabbit antibody (1:250 dilution; Abcam) and a secondary Alexa Fluor 680 goat anti-rabbit antibody (1:20,000 dilution; Invitrogen, Grand Island, NY). For detection of β-actin protein (loading control), a primary monoclonal antibody (1:1000 dilution; Sigma/Aldrich, St Louis, MO) and a secondary 800 nm goat anti-mouse antibody (1:15,000 dilution; LI-COR Biosciences) were used. Primary and secondary antibodies were diluted in Odyssey blocking buffer plus 0.1% Tween-20.
Detection and analysis of MUC5AC, MUC5B, and AGR2 signals were performed using the Odyssey infrared imaging system (LI-COR Biosciences).
Overexpression of IRE1β, spliced XBP-1, and DN-XBP-1. Human IRE1β cDNA clones were purchased from Open Biosystems and cloned into the retroviral vector pQCXIN (Clontech). The spliced XBP-1 and DN-XBP-1 cDNA clones have been previously described.5 Generation of stable Calu-3 cell lines expressing IRE1β, XBP-1s, or DN-XBP-1 followed a published method.5
Mucin secretion studies. Baseline secretion of MUC5AC was evaluated as previously reported,32 with 7- to 10-day-old cultures. Briefly, secreted MUC5AC was collected after a 2-h incubation of Calu-3 cell cultures with HANKS-buffered saline solution (containing 1.6 mM Ca2+, 1.8 mM Mg2+, and 20 mM HEPES) at 37 °C, and vacuum-blotted onto nitrocellulose membranes using a slot-blot apparatus (Biorad, Hercules, CA). Secreted MUC5AC was labeled using the monoclonal antibody 45M1 (Thermo Scientific) at 1:500 dilution and a 800 nm goat anti-mouse secondary antibody at 1:15,000 dilution in Odyssey blocking buffer (LI-COR Biosciences), and detected using the Odyssey infrared imaging system (LI-COR Biosciences).
XBP-1 mRNA splicing. In vivo studies: Mice underwent the same OVA sensitization protocol and were challenged with OVA on days 24 and 25. Tracheas from WT and Ire1β−/− mice were collected on day 26 for RNA isolation. XBP-1 mRNA splicing was assessed as previously reported,53 using the following mouse primer sequences: 5′-ACACGCTTGGGAATGGACAC-3′ (forward) and 5′-CCATGGGAAGATGTTCTGGG-3′ (reverse). Data were quantified with a Fluor Chem Q system (Protein Simple, Santa Clara, CA).
In vitro studies: Calu-3 cells were exposed to serosal IL-13 for different amounts of time and washed with phosphate-buffered saline before the RNA isolation. The assessment of XBP-1 mRNA splicing was performed as previously described.4, 5 Data were quantified with a Storm phosphorimaging system (GE Healthcare Biosciences, Piscataway, NJ).
Statistical analyses. In vivo studies with WT, IRE1β+/−, and IRE1β−/− are representative of three to five tissues or individual experiments. Images from murine airways are representative of several sections/animal. Images for the immunolocalization of IRE1β in normal and CF human airways are representative of several sections from three tissue codes. Data from normal and asthmatic human airway epithelia are from four tissue codes. Data from time courses using primary cultures of HBE are from two individual experiments performed in duplicate. Data from Calu-3 cell cultures are representative of three to four individual experiments. Statistical analyses were performed using either one-way analysis of variance, including standard (parametric) methods and the Tukey–Kramer multiple comparisons test (for three or more experimental groups) or two-tail P-value unpaired t-test (for two experimental groups). Data from bar graphs represent the mean±s.e.m. and statistical significance was defined with P<0.05.
References
Kirkham, S., Sheehan, J.K., Knight, D., Richardson, P.S. & Thornton, D.J. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J. 361 (Part 3), 537–546 (2002).
Morcillo, E.J. & Cortijo, J. Mucus and MUC in asthma. Curr. Opin. Pulm. Med. 12, 1–6 (2006).
Ribeiro, C.M.P., Paradiso, A.M., Carew, M.A., Shears, S.B. & Boucher, R.C. Cystic fibrosis airway epithelial Ca2+i signaling. The mechanism for the larger agonist-mediated Ca2+i signals in human cystic fibrosis airway epithelia. J. Biol. Chem. 280, 10202–10209 (2005).
Ribeiro, C.M.P. et al. Chronic airway infection/Inflammation Induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J. Biol. Chem. 280, 17798–17806 (2005).
Martino, M.E.B., Olsen, J.C., Fulcher, N.B., Wolfgang, M.C., O'Neal, W.K. & Ribeiro, C.M.P. Airway epithelial inflammation-induced endoplasmic reticulum Ca(2+) store expansion is mediated by X-box binding protein-1. J. Biol. Chem. 284, 14904–14913 (2009).
Kelsen, S.G., Duan, X., Ji, R., Perez, O., Liu, C. & Merali, S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am. J. Respir. Cell Mol. Biol. 38, 541–550 (2008).
Kaufman, R.J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233 (1999).
Patil, C. & Walter, P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13, 349–355 (2001).
Lee, A.S. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem. Sci. 26, 504–510 (2001).
Kaufman, R.J., Scheuner, D., Schroeder, M., Shen, X., Lee, K., Liu, C.Y. et al. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell. Biol. 3, 411–421 (2002).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Urano, F., Bertolotti, A. & Ron, D. IRE1 and efferent signaling from the endoplasmic reticulum. J. Cell Sci. 113 (Part 21), 3697–3702 (2000).
Iwawaki, T., Akai, R., Yamanaka, S. & Kohno, K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc. Natl Acad. Sci. USA 106, 16657–16662 (2009).
Ghosh, R. et al. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One 5, e9575 (2010).
Bertolotti, A. et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J. Clin. Invest. 107, 585–593 (2001).
Heazlewood, C.K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Day, A., Carlson, M.R., Dong, J., O'Connor, B.D. & Nelson, S.F. Celsius: a community resource for Affymetrix microarray data. Genome Biol. 8, R112 (2007).
Chen, G. et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 119, 2914–2924 (2009).
Park, K.S. et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 117, 978–988 (2007).
Park, S.W. et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl Acad. Sci. USA 106, 6950–6955 (2009).
Schroeder, B.W. et al. AGR2 Is Induced in Asthma and promotes allergen-induced mucin overproduction. Am. J. Respir. Cell Mol. Biol. 47, 178–185 (2012).
Long, A.J. et al. Gob-5 contributes to goblet cell hyperplasia and modulates pulmonary tissue inflammation. Am. J. Respir. Cell Mol. Biol. 35, 357–365 (2006).
Patel, A.C., Brett, T.J. & Holtzman, M.J. The role of CLCA proteins in inflammatory airway disease. Annu. Rev. Physiol. 71, 425–449 (2009).
Bernacki, S.H. et al. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and muc5b are strongly induced. Am. J. Respir. Cell Mol. Biol. 20, 595–604 (1999).
Holmen, J.M. et al. Mucins and their O-Glycans from human bronchial epithelial cell cultures. Am. J. Physiol. 287, L824–L834 (2004).
Perez-Vilar, J., Ribeiro, C.M., Salmon, W.C., Mabolo, R. & Boucher, R.C. Mucin granules are in close contact with tubular elements of the endoplasmic reticulum. J. Histochem. Cytochem. 53, 1305–1309 (2005).
Evans, C.M. et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol. 31, 382–394 (2004).
Tuvim, M.J. et al. Synaptotagmin 2 couples mucin granule exocytosis to Ca2+ signaling from endoplasmic reticulum. J. Biol. Chem. 284, 9781–9787 (2009).
Kreda, S.M. et al. Characterization of wild-type and {Delta}F508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol. Biol. Cell 16, 2154–2167 (2005).
Kreda, S.M. et al. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J. Physiol. (Lond. ) 584 (Part 1), 245–259 (2007).
Reimold, A.M. et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307 (2001).
Shaffer, A.L. et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21, 81–93 (2004).
Sriburi, R., Jackowski, S., Mori, K. & Brewer, J.W. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell. Biol. 167, 35–41 (2004).
Ron, D. & Hampton, R.Y. Membrane biogenesis and the unfolded protein response. J. Cell. Biol. 167, 23–25 (2004).
Lee, A.H., Chu, G.C., Iwakoshi, N.N. & Glimcher, L.H. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368–4380 (2005).
Ribeiro, C.M. & Boucher, R.C. Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc. Am. Thorac. Soc. 7, 387–394 (2010).
Lee, A.H., Iwakoshi, N.N. & Glimcher, L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 23, 7448–7459 (2003).
Greene, C.M. & McElvaney, N.G. Protein misfolding and obstructive lung disease. Proc. Am. Thorac. Soc. 7, 346–355 (2010).
Marcinak, S.J. & Ron, D. The unfolded protein response in lung disease. Proc. Am. Thorac. Soc. 7, 356–362 (2010).
Cantin, A.M. Cellular response to cigarette smoke and oxidants: adapting to survive. Proc. Am. Thorac. Soc. 7, 368–375 (2010).
Iwawaki, T. et al. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat. Cell Biol. 3, 158–164 (2001).
Imagawa, Y., Hosoda, A., Sasaka, S., Tsuru, A. & Kohno, K. RNase domains determine the functional difference between IRE1alpha and IRE1beta. FEBS Lett. 582, 656–660 (2008).
Nakamura, D., Tsuru, A., Ikegami, K., Imagawa, Y., Fujimoto, N. & Kohno, K. Mammalian ER stress sensor IRE1beta specifically down-regulates the synthesis of secretory pathway proteins. FEBS Lett. 585, 133–138 (2011).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Iqbal, J. et al. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell. Metab. 7, 445–455 (2008).
Volkmann, K. et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J. Biol. Chem. 286, 12743–12755 (2011).
Ribeiro, C.M.P. et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS ONE 4, e5806 (2009).
Zhu, Y. et al. Munc13-2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J. Physiol. (Lond. ) 586, 1977–1992 (2008).
Mall, M.A. et al. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am. J. Respir. Crit. Care Med. 177, 730–742 (2008).
Livraghi, A. et al. Airway and lung pathology due to mucosal surface dehydration in ß-Epithelial Na+ channel-overexpressing mice: role of TNF-alpha and IL-4R-alpha signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J. Immunol. 182, 4357–4367 (2009).
Smith, J.A., Turner, M.J., DeLay, M.L., Klenk, E.I., Sowders, D.P. & Colbert, R.A. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. Eur. J. Immunol. 38, 1194–1203 (2008).
Acknowledgements
We thank Syanne Olson for editorial assistance; Drs Peter Bove and Cedric Weber for cDNA from alveolar type II cells and HBE; Dr Scott Randell for freshly isolated human tissues and HBE cells; Dr Laurie Glimcher for the DN-XBP-1; Kimberly Burns for histological specimens, and AB–PAS and H&E stains; Dr Silvia Kreda for technical advice towards culturing Calu-3 cells; and Dr Hong Dang for advice towards statistical analyses. This work was supported by grants from the Cystic Fibrosis Foundation (RIBEIRO07P0), American Asthma Foundation (10-0203), and the National Heart, Blood and Lung Institute (R21HL104309-01) to CMP Ribeiro, and the National Institute of Diabetes, Digestive and Kidney Diseases (DK047119) to D. Ron. D. Ron is a Principal Research Fellow of the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Martino, M., Jones, L., Brighton, B. et al. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol 6, 639–654 (2013). https://doi.org/10.1038/mi.2012.105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.105
This article is cited by
-
Keeping goblet cells unstressed: new insights into a general principle
The EMBO Journal (2024)
-
The IRE1β-mediated unfolded protein response is repressed by the chaperone AGR2 in mucin producing cells
The EMBO Journal (2023)
-
Evolution and function of the epithelial cell-specific ER stress sensor IRE1β
Mucosal Immunology (2021)
-
NeTFactor, a framework for identifying transcriptional regulators of gene expression-based biomarkers
Scientific Reports (2019)
-
Causes and consequences of endoplasmic reticulum stress in rheumatic disease
Nature Reviews Rheumatology (2017)