Abstract
The human gut mucosa is a major site of human immunodeficiency virus (HIV) infection and infection-associated pathogenesis. Increasing evidence shows that natural killer (NK) cells have an important role in control of HIV infection, but the mechanism(s) by which they mediate antiviral activity in the gut is unclear. Here, we show that two distinct subsets of NK cells exist in the gut, one localized to intraepithelial spaces (intraepithelial lymphocytes, IELs) and the other to the lamina propria (LP). The frequency of both subsets of NK cells was reduced in chronic infection, whereas IEL NK cells remained stable in spontaneous controllers with protective killer immunoglobulin-like receptor/human leukocyte antigen genotypes. Both IEL and LP NK cells were significantly expanded in immunological non-responsive patients, who incompletely recovered CD4+ T cells on highly active antiretroviral therapy (HAART). These data suggest that both IEL and LP NK cells may expand in the gut in an effort to compensate for compromised CD4+ T-cell recovery, but that only IEL NK cells may be involved in providing durable control of HIV in the gut.
Similar content being viewed by others
Introduction
Over the course of the past decade, our understanding of human immunodeficiency virus (HIV) pathogenesis has changed considerably, as we have begun to appreciate the role of the gastrointestinal (GI) tract in HIV-associated immunopathology.1, 2, 3 Several lines of evidence suggest that HIV preferentially infects activated memory CD4+ T cells,4 and therefore the gut, which harbors a large concentration of activated memory CD4+ T cells and provides fertile ground for rapid HIV infection and dissemination. As early as the first few weeks of HIV infection, significant physical and immunological changes occur in the gut, including a massive accumulation of CD8+ T lymphocytes2, 5, 6 and a massive depletion of CD4+ T cells.7 These dramatic changes in cell frequencies in the gut are accompanied by increased gut permeability resulting in microbial translocation, leading to elevated levels of microbial products in the blood such as lipopolysaccharide that has been shown to contribute to the induction of immune activation.8
Despite the early burst of HIV replication in acute infection, in both the blood and the gut, acute viral replication is brought down to a viral set point that persists throughout chronic infection.9 Given that HIV replicates robustly in the GI tract, it is likely that immune responses must work aggressively during acute infection in the gut to contain the ongoing dramatic viral replication. Therefore, it is plausible that these GI antiviral responses may represent the most robust control over the virus, and measurements of the immune response in the peripheral blood may not necessarily reflect responses in the gut.
Interestingly, epidemiological and functional evidence suggest that natural killer (NK) cells have an important role in controlling acquired immunodeficiency syndrome progression.10, 11, 12 Highly functional clonal populations of NK cells that expand rapidly during acute HIV infection, before induction of adaptive immune responses10, 11 and particular killer immunoglobulin-like receptors (KIRs), namely KIR3DS1 and KIR3DL1, which interact with a subclass of human leukocyte antigen (HLA)-B alleles, called HLA-Bw4-80I, are associated with slower HIV-1 disease progression.13, 14 Thus, increasing evidence points to an important role for NK cells in early and durable antiviral control; however, the mechanism(s) and location of their antiviral activity remain unknown.
NK cells are large granular lymphocytes that have a major role in the elimination of both tumors and virally infected cells without the need for antigen sensitization.15 Several lines of evidence suggest that dramatic changes occur within the NK cell compartment during HIV infection, including phenotypic and functional changes16, 17, 18, 19 that potentially contribute to the failure to control infection and progression to acquired immunodeficiency syndrome.20, 21 However, NK cells in individuals expressing protective KIR/HLA genotypes expand rapidly in acute infection and exhibit strong antiviral activity against HIV in vitro.14 Furthermore, recent studies from Vieillard et al.22 demonstrated that spontaneous HIV controllers exhibit robust NK cell responses. Yet, given that the majority of HIV viral replication occurs in the gut, it is essential to define whether gut mucosal NK cells contribute to antiviral control at this vulnerable site of infection in these unique individuals that control HIV infection in the absence of therapy.
Early studies have suggested that NK cells reside in the intraepithelial space of the GI tract and in fact, NK cells have been identified as intraepithelial lymphocytes (IELs) in various species of animals23, 24, 25, 26 and humans.27 In humans, gut NK cells, similar to uterine NK cells, express high levels of CD56, KIR, and CD16,28 produce proinflammatory cytokines (interferon-γ and tumor necrosis factor-α), and kill major histocompatibility class-negative target cells (K562 cells) in the presence of interleukin (IL)-2, IL-12, or IL-15.29 Furthermore, gut NK cells have been shown to be crucially involved in controlling murine enteric coronaviral infections.30 More recent data have documented a new subset of NK cells or lymphoid tissue inducers, NK-22 cells that secrete IL-22, which is required for the maintenance of epithelium integrity.31, 32 Yet, little is known about the distribution of NK cells in the human gut, and how these change during viral infections.
Here, we sought to define whether durable control of HIV infection was associated with the preferential recruitment of NK cell populations to the gut, by analyzing rectosigmoid mucosal biopsies collected from patients at various stages of HIV infection. In our studies, we identified two distinct populations of gut mucosal-resident NK cells, one present in the IEL (IEL NK) and the other in the lamina propria (LP NK). Interestingly, we observed changes in IEL and LP NK cell frequencies in HIV infection. Unexpectedly, increased frequencies of both IEL and LP NK cells were only observed in subjects with incomplete peripheral blood CD4+ T-cell recovery (CD4 <350 cells per μl) despite long-term antiretroviral therapy (ART)-mediated viral suppression. However, importantly, spontaneous controllers expressing protective KIR/HLA genotypes had higher IEL NK cell numbers than did those with non-protective genotypes, suggesting that IEL NK cells may contribute to durable control of HIV in these individuals. These data suggest that two subsets of NK cells exist in the human GI tract, one in the IEL and the other in the LP, one of which may be involved in durable control of HIV infection, and both that may expand at this vulnerable site in response to incomplete immune recovery after virologic suppression.
Results
GI tract CD3negNKp46pos NK cells are localized within the intraepithelial space and LP
Epidemiological data suggest that NK cells may have a critical role in controlling HIV-1 infection;13 however, the majority of studies have focused on NK cells in the blood, whereas the virus replicates most profusely in the gut.3 Although several reports suggest that NK cells can be detected in the gut mucosa in animals,23, 24, 25, 26, 33, 34, 35 less is known about NK cells in the human gut. Thus, to define the frequency of NK cells in the gut, as well as their distribution and localization, immunohistochemistry (IHC) was used to visualize and quantify these cells. Traditionally, NK cells are defined as CD3negCD56pos/negCD16pos/neg; however, this combination of markers is difficult to use in immunohistochemical staining because of the fact that CD56 also stains the neural tissue that is abundant in the GI tract. In contrast, specific family of receptors, the natural cytotoxicity receptors, are believed to be exclusively expressed on NK cells.36 Thus, NKp46, an natural cytotoxicity receptor expressed on nearly all NK cells, was selected. Two NKp46 antibodies (Abs) were used in the study to detect NK cells in the human colon: polyclonal goat anti-human (GAH) NKp46 and monoclonal mouse anti-human (MAH) NKp46. Unexpectedly, these two Abs labeled two distinct cell populations in the gut. The GAH NKp46 exclusively labeled cells located in intraepithelial spaces (IEL NK) (Figure 1a and c), whereas MAH NKp46 labeled LP cells (LP NK) (Figure 1b and d). LP NK cells were located in the basolateral part of the mucosa and within the gut submucosa. Neither of these Abs labeled cells within lymphoid aggregates, suggesting that NK cells may be excluded from inductive sites in the gut (data not shown). In addition, a subset of IEL NK cells (Figure 2a), but not LP NK cells (Figure 2b), co-expressed CD57, a marker of terminal differentiation, present on NK and T cells,37 suggesting that these two NK cell subsets represent phenotypically and potentially functionally distinct cell types. Thus, two populations of NK cells were identified in the healthy human gut, located in distinct regions of the tissues, potentially reflecting unique functional characteristics.
Two distinct subsets of gut NK cells exist in different regions of the human colon. Immunohistochemical (IHC) staining depicts (a) IEL NK cells stained in brown with GAH NKp46 antibody and (b) LP NK cells stained in brown with MAH NKp46 antibody. To confirm that NKp46+ cells were not T cells, double staining IHC was performed with CD3 in brown and (c) GAH NKp46 and (d) MAH NKp46 in red. All images are displayed at original magnification × 400. GAH, goat anti-human; IEL, intraepithelial lymphocyte; LP, lamina propria; NK, natural killer.
IEL, but not LP, NK cells express CD57. Multicolor confocal immunofluorescence images show co-expression of CD57 (white), a marker of differentiated NK cells, on a subset of (a) IEL NK cells defined as CD3-negative (red) and NKp46-positive (green). (b) LP NK cells showed no such co-expression of CD57. Both images are displayed at original magnification × 630. IEL, intraepithelial lymphocyte; LP, lamina propria; NK, natural killer.
Confirmation of NKp46 Ab specificity
Having identified two populations of NK cells in the gut stained with different NKp46 Abs, we next sought to confirm that these IHC Abs labeled NK cells in other tissues as well. We investigated whether these Abs recognized NK cells in the uterine tissue, known to be abundant in NK cells.38 Abundant levels of NKp46+ cells were identified in healthy endometrial tissues with both GAH and MAH NKp46 Abs (Figure 3a and b), suggesting that these Abs did in fact recognize NK cells at other mucosal sites.
NKp46 antibodies also stain NK cells in the uterus. Both (a) the GAH and (b) the MAH NKp46 antibodies labeled NK cells (brown) in the endometrial section. All images are displayed at original magnification × 200. GAH, goat anti-human; MAH, mouse anti-human; NK, natural killer.
To further confirm that NKp46 Abs labeled CD3neg cells, we performed a double staining by IHC and multicolor immunofluorescence to simultaneously label CD3pos and NKp46pos cells. Neither of the NKp46pos cell subsets stained positively with the CD3 Ab, confirming that neither IEL nor LP NK cells belong to the T-cell lineage (Figures 1c, d and 2a, b).
To ultimately validate that these NKp46 Abs labeled NK cells specifically, both NKp46 Abs were tested by flow cytometry and compared with the standard BD NKp46 Ab (clone 9E2) widely used to stain peripheral blood NK cells. Both GAH and MAH NKp46 stained similar frequencies of CD3negCD56pos/negCD16pos/neg blood NK cells as did the traditional BD Ab (Figure 4a). Moreover, these Abs did not label other cell subsets (data not shown). To further confirm the specificity of these Abs for NK cells, NK cells were also costained with a range of additional NK cell markers, including NKp30, NKG2A, KIR, and NKG2D (Figure 4a), demonstrating that both GAH and MAH NKp46 Abs labeled similar frequencies of NK cells bearing these other phenotypic markers.
Flow cytometric confirmation of NKp46 antibody specificity for NK cells in the peripheral blood. To confirm that the two NKp46 antibodies specifically stained NK cells, the specificity of the antibodies was verified by flow cytometry using a panel of NK cell-specific antibodies. The overall frequency of CD3−CD16+/−CD56+/− NK cells stained with the traditional BD NKp46, the GAH NKp46, and the MAH NKp46 were similar. (a) Additional NK cell markers, including NKp30, NKG2A, KIR, NKG2D, were expressed at equivalent levels on blood NK cells labeled with the three different NKp46 antibodies. (b) Furthermore, lymphocytes isolated from the gut tissue were stained with the traditional BD NKp46, the GAH NKp46, and the MAH NKp46 to confirm that these antibodies label CD45posCD3neg NK cells in mucosal samples. GAH, goat anti-human; MAH, mouse anti-human; NK, natural killer.
Finally, to confirm that GAH and MAH NKp46 Abs stain mucosal NK cells, freshly collected enzymatically digested gut tissue was stained with the two Abs, and frequencies of NK cells were compared with the traditional BD Ab NK cell frequencies. All three NKp46 Abs labeled CD45posCD3neg lymphocytes isolated from the gut (Figure 4b), which also co-expressed CD56 and CD16 (data not shown). However, interestingly, the three Abs labeled different frequencies and intensities of CD56pos NK cells, in such a manner that the MAH labeled more CD56bright NK cells than did the GAH NKp46 Ab, suggesting some differences in NK cell-subset specificity in the gut. Furthermore, all three Abs stained CD45posCD3negNKp46pos cells that also co-expressed NKG2A, confirming that the stained mucosal cells belong to the NK cell lineage (Figure 4b). Thus, overall, these data suggest that both NKp46 Abs, used to detect NK cells in the gut, are highly specific for NK cells.
No alteration in the IEL:LP NK cell balance during HIV infection
Given the fact that the two NKp46 Abs labeled two distinct NK cell populations in the gut, we next sought to determine whether HIV infection is associated with differential recruitment of either of these NK cell subsets to this vulnerable site, where they may have a role in antiviral control. Slides from colon biopsies were collected from 65 subjects, including 15 HIV-negative individuals and 50 HIV-positive patients with differential control of HIV infection: 20 untreated controllers (11 elite controllers (ECs): <75 RNA copies per ml and 9 viremic controllers (VCs): 75–2,000 RNA copies per ml), 9 chronic untreated patients (<2,000 RNA copies per ml) and 21 treated patients (11 with optimal CD4+ T-cell recovery and 10 with suboptimal recovery: all <75 RNA copies per ml). Slides were stained with GAH and MAH NKp46 Abs, and frequencies of IEL and LP NK cells were counted manually in whole-tissue scans. Patients were grouped into HIV-negative controls and HIV-infected individuals for initial studies.
In healthy controls IEL NK cells were more frequent than were LP NK cells (Figure 5a). Despite the presence of both of these subsets of NK cells in the gut, there was no relationship between the frequency of each subset in the gut of healthy individuals (Figure 5c), suggesting that they may be recruited independently to these sites. Interestingly, although the ratio of IEL:LP NK cells was preserved in HIV infection (Figure 5b), a positive correlation was observed between these two NK cell populations in HIV+ subjects (Figure 5d), suggesting that in HIV infection, these cells may be synergistically recruited to the gut, to contribute to immune control. These data suggest that under homeostatic conditions, the frequency of IEL and LP NK cells is unrelated, but during infection, the recruitment/expansion of these cells in the gut may occur in a coordinated manner.
IEL NK cells are more abundant than LP NK cells in both healthy and HIV-infected populations. (a) The total frequency of IEL and LP NK cells was counted per mm2 of colon tissue in healthy individuals, demonstrating that IEL NK cells are more abundant in the gut mucosa than LP NK cells **P<0.01. (b) Similarly, IEL NK cells exceeded the frequency of LP NK cells in the colon tissue of HIV-infected individuals **P<0.01. (c) Furthermore, the relationship between IEL and LP NK cells was explored, demonstrating no visible correlation in cell frequencies in healthy individuals, (d) but a significant correlation in HIV+ patients. HIV, human immunodeficiency virus; IEL, intraepithelial lymphocyte; LP, lamina propria; NK, natural killer.
Expansion of NK cells in immunologically non-reconstituted patients after HAART
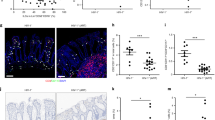
Given the alteration in the relationship between IEL and LP NK cells in HIV infection, we were intrigued to next define whether these cells were specifically expanding/accumulating in specific HIV-infected patient populations. We hypothesized that both of these NK cell subsets would expand preferentially in the controller group, potentially contributing to durable control of HIV infection, whereas we speculated that the frequency of these cells would be depressed in chronically infected individuals off therapy. As expected, we observed a trend toward a decrease in the frequency of both IEL and LP NK cells in chronic untreated patients with high viral loads (VLs) as compared with HIV subjects (Figure 6a and b). However, we observed a remarkably heterogeneous distribution of NK cells in controllers, marked by a wide distribution of both IEL and LP NK cell frequencies, with some individuals exhibiting some of the highest levels of both subsets of NK cells in their guts, whereas the vast majority exhibited some of the lowest frequencies of these cells. No difference was observed in either IEL or LP NK cell frequencies among ECs compared with VCs (Figure 6c). Conversely, the most dramatic expansion of both IEL and LP NK cells was observed in the treated patient group that exhibited incomplete or suboptimal CD4+ T-cell recovery despite long-term ART (immunological non-responders (INRs)) (ART INR:CHRO UNTX P<0.05 for both IEL and LP NK cells, Figure 6a and b). LP NK cells in INRs were also significantly more frequent compared with LP NK cells in spontaneous controllers (ART INR:CONTRL P<0.05). Interestingly, this expansion of IEL and LP NK cells in INR patients was related (Figure 6d), and seemed to drive the IEL/LP NK cell correlation observed in Figure 5d. These data suggest for the first time that NK cell distribution in the gut of controllers is quite heterogeneous, NK cell frequencies decline in chronic HIV infection, but that both IEL and LP NK cells expand in the GI tract of incompletely reconstituted HIV+ subjects on ART, suggesting that NK cells may accumulate in the absence of viral replication in this vulnerable mucosal tissue in response to incomplete immunological reconstitution, to potentially provide a compensatory mechanism for the compromised immunity.
Frequency changes in IEL and LP NK cells in HIV+ patients with differential control of infection and healthy controls. The frequency of (a) IEL and (b) LP NK cells was compared among the different HIV+ patient populations, including spontaneous controllers (CONTRL), chronic untreated patients (CHRO UNTX), treated chronically infected patients who exhibited immunological reconstitution following HAART (ART+IR), treated chronically infected patients who were immunological non-responders (ART+INR), and healthy controls (HIV−); *P<0.05. These data demonstrate the expansion of both IEL and LP NK cells in ART-INR patients. (c) Furthermore, the frequency of IEL and LP NK cells were also compared among elite (EC) and viremic (VC) controllers. (d) IEL and LP NK cells frequencies are correlated in the INR group. The relationship between the frequency of IEL NK cells and the percentage CD4+ T cells (CD4 PCT) and the percentage CD8+ T cells (CD8 PCT) in the periphery is illustrated in e. ART, antiretroviral therapy; HIV, human immunodeficiency virus; IEL, intraepithelial lymphocyte; INR, immunological non-responsive; LP, lamina propria; NK, natural killer.
Positive correlation of gut IEL NK cells and CD4 T cells in periphery in chronic infection
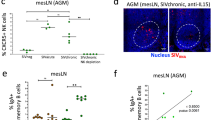
Given that the absolute number of circulating CD4+ T cells and plasma viral set point serve as markers of disease progression, we were next interested in defining whether the frequencies of either of the two populations of NK cells in the gut of HIV-infected patients were related to changes with either of these two clinical parameters. Therefore, we compared the frequency of NK cells with the VL, CD4, and CD8 count in the peripheral circulation. We found no correlation between VL and NK cells in any of the patient populations, potentially suggesting that the peripheral level of viremia may not drive these changes in NK cell frequencies, but that perhaps local viral replication could have an impact. However, a positive correlation between IEL NK cells and peripheral blood CD4+ T cells (R2=0.3322, P=0.0498) and a negative correlation between IEL NK and peripheral blood CD8+ T cells (R2=0.4071, P=0.0256) was observed in untreated, chronically infected patients (Figure 6d). These data show that inversion of the CD4:CD8 ratio, associated with inflammation, may potentially reflect a dampening of the NK cell response in the setting of an increase in CD8+ T-cell numbers, as has been previously shown in acute HIV infection.11
Higher frequency of IEL NK cells in spontaneous controllers with protective KIR/HLA genotypes
Previous epidemiological studies have shown that particular KIR/HLA compound genotypes are associated with spontaneous control of HIV.13, 39 Given the wide variation in NK cell frequencies in spontaneous controllers (Figure 6a and b), unrelated to the degree of antiviral control (Figure 6c), we next sought to determine whether these protective KIR/HLA genotypes may account for differences in NK cell frequencies in the gut in spontaneous controllers. Thus, ECs and VCs were genotyped for KIR and HLA, and were grouped into those with protective genotypes (KIR3DS1 or KIR3DL1 *001, *002, *008, *015, and *009 in combination with HLA-Bw4 alleles with an isoleucine at position 80) and those without protective genotypes (the rest). Interestingly, there was a trend toward significantly higher frequencies of IEL NK cells detected in controllers with protective KIR/HLA compound genotypes (P=0.0549) but not in LP NK cells (Figure 7). These data support a potential role for a persistent expansion of protective IEL NK cell populations in the gut mucosa in spontaneous controllers that may directly contribute to durable control of viral replication at this vulnerable site beyond acute infection.
Impact of KIR/HLA compound genotypes on the frequency of gut mucosal NK cells. The difference in numbers of IEL NK cells and LP NK cells was compared between controllers expressing protective KIR/HLA alleles (KIR3DS1-Bw4-80I or KIR3DL1*h-Bw4-80I) with those not expressing protective KIR/HLA alleles. HLA, human leukocyte antigen; IEL, intraepithelial lymphocyte; KIR, killer immunoglobulin-like receptor; LP, lamina propria; NK, natural killer.
Discussion
On the basis of our recent understanding of the role of the gut as the primary site of HIV viral replication, we sought to define whether NK cells, a subset of innate immune cells, may accumulate preferentially at this site in individuals who progress more slowly to disease. In our study, we identified two populations of NK cells in the GI tract, one that resides in intraepithelial space of the crypts (IEL NK) and the other present in the LP (LP NK) of the colon tissue (Figure 1). Furthermore, we observed a remarkable heterogeneity in the distribution of IEL and LP NK cells in spontaneous controllers, in which some of these patients expressed the highest frequencies of NK cells in the gut, but the majority exhibited reduced frequencies of these innate immune cells, similar to levels observed in chronic HIV infection. Moreover, we observed that the frequency of both IEL and LP NK cells were expanded in HAART-treated patients who did not fully reconstitute their CD4+ T-cell numbers (Figure 6a and b). In addition, IEL NK cells were preserved in spontaneous controllers with protective KIR/HLA genotypes compared with controllers with non-protective KIR/HLA genetic backgrounds. These data suggest for the first time that IEL NK cells may contribute to persistent durable control of viral replication in the gut in controllers with protective KIR/HLA compound genotypes, and that both IEL and LP NK cells expand at this vulnerable site in individuals who are incompletely able to recover from the assault of the virus, potentially in an effort to compensate for the persisting compromised environment within this barrier.
It is incompletely understood what contributes to the lack of immunological reconstitution. Some recent data show that collagen deposition and disruption of the lymphatic tissue architecture contribute to persistent CD4+ T-cell depletion before treatment, and that the extent of these changes affects the efficiency of immune reconstitution after the initiation of therapy.40 Such observations have been made at first in lymph nodes40 and subsequently in gut-associated lymphatic tissue,41 the latter suffering from a more pronounced loss in CD4+ T-cell numbers.3, 42, 43, 44 Furthermore, INRs exhibit particular unique clinical characteristics, including fibrosis in lymph nodes and gut tissue, increased persistent immune activation, and elevated markers of chronic inflammation despite undetectable VLs.45 Thus, despite the direct antiretroviral effects on reducing viral replication, these individuals still exhibit some markers of chronic disease. In addition to these markers of continued disease pathology, we report here that these individuals also exhibit a remarkable expansion of NK cells in the gut (Figure 6a and b). We speculate that incomplete immunological reconstitution causes some additional inflammatory stimuli in the gut, potentially due to some persistent low-level viral replication, resulting in the persistent recruitment of innate immune cells to help provide some level of protection in the absence of CD4+ T cells. Thus, mucosal NK cells may expand in an effort to compensate for the compromised gut mucosal immunity. Interestingly, this NK cell expansion is not observed in chronic untreated patients, despite the fact that they exhibit reduced CD4+ T-cell numbers as well; however, these individuals also exhibit high levels of viral replication and persistent immune activation, due to microbial translocation,8 through accessory cells.46 However, this hyperactivation may drive direct NK cell apoptosis,47 thereby preventing the observed increase of these cytolytic effector cells within tissues.
Epidemiological work strongly suggests that NK cells may be directly involved in antiviral control of HIV.10, 13, 14 Therefore, we speculated that these innate effectors may expand preferentially in the gut of individuals that exhibit durable control of HIV, including ECs and VCs. Overall controllers exhibited a heterogeneous distribution of both IEL and LP NK cells in mucosal sections (Figure 6a and b), which did not differ in ECs or VCs (Figure 6c), and were similar in overall numbers from that seen in chronically infected individuals. However, given the strong epidemiological data suggesting that specific KIR/HLA combinations contribute to spontaneous control of HIV infection, we examined IEL and LP NK cell distributions among controllers with protective and non-protective KIR/HLA genotypes. Interestingly, we observed a trend toward an elevated IEL, but not LP, NK cells in controllers with protective KIR/HLA genotypes, suggesting for the first time that particular NK cell populations may persist in these unique individuals at this vulnerable site, aimed at maintaining long-term control of HIV infection. The specific preservation of IEL, and not LP, NK cells may reflect functional differences between these subsets of lymphocytes. Previous work has suggested that lymphocytes able to traffic in the IEL exhibit more cytolytic functional properties than those found in the LP.48 This may suggest that elevated IEL NK cells in controllers with protective KIR/HLA genotypes may confer an enhanced capacity to rapidly deploy the cytolytic antiviral activity of NK cells, should the cell encounter a potential viral burst that have been suggested to occur in some controllers.49 However, additional in-depth functional studies are required to begin to dissect the functional and phenotypic properties of NK cells in these two compartments of the tissues, and particularly those that may expand in spontaneous control in the setting of protective KIR/HLA genotypes.
Early studies from animal models have shown IEL lymphocytes to have higher cytolytic activity than LP48 or Peyer's patches lymphocytes;35 however, the specific distribution and functional properties of NK cells in the gut have been poorly explored. A population of cells, called NK-22, was reported to reside in the LP of tonsils, lacking classical markers (including NKp46) of NK cells, but expressing NKp44 and secreting IL-22 required for the maintenance of epithelium integrity.31 Yet, several lines of evidence now suggest that NK-22 cells belong to a subset of lymphoid tissue-inducer cells, rather than conventional NK cells.32 However, here, we report the presence of two NK cell subsets, distinct from NK-22 as they express NKp46, which are distributed in distinct effector compartments of the gut. On the basis of their location within the mucosa, we speculate that IEL NK cells may be more cytolytic and may serve as the first line of defense, just beneath the epithelial border, whereas LP NK cells, located in the basolateral surface of the gut mucosa and in the submucosa may constitute an immunoregulatory type of NK subset, similar to that seen in the uterine tissues. These two NK cell subsets exhibit distinct phenotypes that support this potential functional difference, as only IEL NK cells co-express the terminal differentiation marker CD57 (Figure 2) that is expressed on more cytolytic blood NK cells.37 The fact that these two NK cell populations were identified by two Abs targeting the same antigen mounted in different species, suggests that the NKp46 receptor may exhibit conformational changes depending on the activation status of NK cells. A similar scenario has been observed for the CD43 molecule, in which two distinct Abs recognize differentially glycosylated isoforms of the receptor, reflecting distinct activation states of the lymphocytes on which they are expressed.50 Given that NKp46 is highly glycosylated,51 we speculate that these two Abs may recognize NKp46 antigens with unique glycosylation patterns or conformational changes on IEL and LP NK cells, providing a useful mechanism by which to identify mucosal NK cell subpopulations.
In summary, our data demonstrate that two distinct populations of NK cells reside in the gut and may represent two innate effector cell subsets with unique functional properties related to their tissue localization. We show that the frequency of both subsets of NK cells change after HIV infection, with an overall loss of both subsets in chronic infection, but with a significant expansion in INRs. Furthermore, we show that the protective KIR/HLA genotypes are associated with a preservation of IEL NK cells in spontaneous controllers. These data suggest that although chronic HIV infection is associated with reduced frequencies of these innate effector cells in the gut, these cells respond robustly and expand in the setting of incomplete immune reconstitution potentially aimed at compensating for incomplete immunological recovery in patients on HAART and in spontaneous controllers with protective KIR/HLA genotypes that may provide durable protection from infection after acute infection.
Methods
Subjects
Formalin-fixed-paraffin-embedded tissue slides from rectosigmoid mucosal biopsy samples obtained from a total of 65 individuals from the Study of the Consequences of the Protease Inhibitor Era (SCOPE) cohort were used in this study, among which 50 were HIV-infected patients and 15 were uninfected healthy controls. HIV-infected samples included 20 untreated HIV spontaneous controllers, who spontaneously control HIV viral replication in the absence of therapy (11 “ECs” with VL <75 copies per ml and 9 “VCs” with VL 75–2,000 copies per ml), 9 untreated non-controllers (“chronic untreated”), who remained off therapy during infection with VL >2,000 copies per ml (most with VL >10,000 copies per ml), and 21 treated patients on ART with VL <75 copies per ml (11 “immunological responders” with optimal CD4 recovery >500 cells per mm3 of blood and 10 INRs with suboptimal CD4+ T-cell recovery <350 cells per mm3). Immunological responders and INRs maintained ART-mediated viral suppression for a median of 8.2 and 4.4 years, respectively. Detectable episodes of viremia <500 copies per ml were allowed during these intervals if preceded and followed by undetectable values. In addition to having lower CD4+ T-cell counts at the time of the rectal biopsy (<350 cells per mm3 vs. >500 cells per mm3), INRs also had a lower median rate of CD4+ T-cell recovery in the 2 years before rectal biopsy (+11.9, interquartile range: +8.2 to +15.2 CD4+ T cells per mm3 per year) than immunological responders (+24.8, interquartile range: +18.3 to +36.7 CD4+ T cells per mm3 per year, P=0.002 for between group comparison). Laboratory personnel were blinded to HIV disease status until measurements were completed.
KIR/HLA genotyping
We performed genotyping of the HLA locus following the PCR-sequence-specific oligonucleotide probing typing protocol recommended by the 13th International Histocompatibility Workshop (http://www.ihwg.org/). For KIR3DL1/S1 subtyping, polymorphic exons 3, 4, 5, 7, 8, and 9 were selectively amplified in four PCR reactions using locus-specific primers (a separate PCR for each of exons 3, 4, and 5, and a fourth PCR for exons 7, 8, and 9 combined). PCR products were blotted on nylon membranes and hybridized with a panel of 56 sequence-specific oligonucleotide probes designed to detect unique sequence motifs of known KIR3DL1 alleles. KIR3DL1 alleles were assigned by the reaction patterns of the sequence-specific oligonucleotide probes based on the known KIR3DL1 sequences, as described previously.39 Ambiguous sequence-specific oligonucleotide probe typing results were resolved by sequencing analysis.
Immunohistochemistry
Formalin-fixed-paraffin-embedded slides were de-paraffinized and hydrated. Antigen retrieval was performed in Deckloaking Chamber (Biocare Medical, Concord, CA), inducing temperature of 125°C and pressure of 25 psi. Endogenous peroxidase activity was blocked with buffered hydrogen peroxide (Biocare Medical) for 5 min at room temperature (RT). Unspecific binding of Abs was blocked using purified casein and other proteins (Background Sniper, Biocare Medical) for 10 min in RT and donkey serum (Sigma, St Louis, MO) for 15 min. Slides were incubated with the following primary Abs for 1 h at RT: mouse monoclonal anti-human NKp46 (R&D Systems, Minneapolis, MN), goat polyclonal anti-human NKp46 (R&D Systems), rabbit polyclonal anti-human CD3 (Dako, Carpinteria, CA), mouse monoclonal anti-human CD57 (Dako). The following control Abs were used: mouse IgG2B isotype control (R&D Systems), goat control Ab (R&D Systems), mouse IgM isotype control (Dako). EnVision+Systems-horseradish peroxidase (anti-mouse and anti-rabbit) or rabbit anti-goat-horseradish peroxidase Ab (Dako) were used for 45 min at RT as a secondary Ab and visualization was performed with a DAB+ (diaminobenzidine) substrate chromogen (Dako). For double staining procedure, denaturing solution, AP-conjugated secondary Abs, and Vulcan Fast Red chromogen (Biocare Medical) were used for detecting the second antigen. Sections were counterstained with hematoxylin, dehydrated, and mounted in mounting medium.
Immunofluorescence
De-paraffinization, hydration, and antigen-retrieval steps were performed as described in the “Immunohistochemistry” section. Before incubation with the primary Ab, slides were immersed in 0.3% Sudan Black B in 70% ethanol for 2 h at RT to mask autofluorescence present in tissues. Slides were blocked and incubated with primary or control Abs as described in the “Immunohistochemistry” section. The following secondary Abs were used for detection of cells: donkey anti-mouse AlexaFluor 555 or A488 (Invitrogen, Carlsbad, CA), donkey anti-goat AlexaFluor A488 (Invitrogen), and donkey anti-rabbit AlexaFluor A647 (Invitrogen). Nuclei were stained with Hoechst 33258 (Invitrogen). Slides were mounted with Gold ProLong antifade reagent (Invitrogen).
Microscopy
Slides were scanned using the Zeiss MIRAX MIDI automated slide scanner (Zeiss, Thornwood, NY) in brightfield mode for IHC staining or fluorescence mode for immunofluorescence staining. High-quality, high-magnification images were taken using the Zeiss LSM510 laser scanning confocal microscope.
Image analysis
Cells were counted manually from 12-bit TIFF images acquired from whole-tissue scans. Tissue area measurements were performed using ImageJ software (NIH, Bethesda, MD) and obtained frequencies of cells were presented as number of cells per mm2 of tissue.
Flow cytometry
Flow cytometric evaluation of NKp46 Ab staining was performed on peripheral blood mononuclear cells and freshly isolated lymphocytes from enzymatically digested GI tissues. At least 106 peripheral blood mononuclear cells or gut lymphocytes were stained with anti-CD3-PacificBlue, anti-CD16-APC-Cy7, anti-CD56-PE-Cy7 (BD Biosciences, San Jose, CA), anti-CD158a/anti-CD158b/anti-NKB1-FITC, anti-NKG2D-PE (BD Biosciences), anti-NKG2A-Alexa Fluor 647 (Beckman Coulter, Fullerton, CA) and one of three NKp46 Abs followed by a secondary conjugated to Alexa Fluor 488 or Alexa Fluor 647. Cells were then washed and fixed by 4% paraformaldehyde, before acquisition on a BD LSRII. The expression of NKp46 was quantified on CD3negCD56pos/negCD16pos/neg lymphocytes using FlowJo (Tree Star, Ashland, OR). In addition, CD45-Pe-Cy5.5 (BD Biosciences) and LIVE/DEAD stain (Invitrogen) were added for tissue lymphocyte staining and the expression of NKp46 was quantified on live, CD45posCD3negCD56pos/negCD16pos/neg lymphocytes.
Statistics
Statistical analyses for comparison of IEL and LP NK cells in healthy and infected groups were performed using a Mann–Whitney U-test. Comparison of protective and non-protective KIR/HLA compound genotypes in spontaneous controller group was performed using a Mann–Whitney U-test. Spearman's rank correlation coefficient was used to examine the relationships between IEL and LP NK cell frequencies. Statistical comparisons of NK cell frequencies among patient groups were made using the Kruskal–Wallis test. P-values of pairwise comparisons were adjusted by using Dunn's method. Reported P-values are two sided and values <0.05 were considered significant.
References
Brenchley, J.M. & Douek, D.C. The mucosal barrier and immune activation in HIV pathogenesis. Curr. Opin. HIV AIDS 3, 356–361 (2008).
Brenchley, J.M. & Douek, D.C. HIV infection and the gastrointestinal immune system. Mucosal. Immunol. 1, 23–30 (2008).
Brenchley, J.M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759 (2004).
Douek, D.C. et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417, 95–98 (2002).
Clayton, F. et al. Rectal mucosal pathology varies with human immunodeficiency virus antigen content and disease stage. Gastroenterology 103, 919–933 (1992).
Rodgers, V.D., Fassett, R. & Kagnoff, M.F. Abnormalities in intestinal mucosal T cells in homosexual populations including those with the lymphadenopathy syndrome and acquired immunodeficiency syndrome. Gastroenterology 90, 552–558 (1986).
Mattapallil, J.J. et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097 (2005).
Brenchley, J.M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 (2006).
Pantaleo, G., Graziosi, C. & Fauci, A.S. Virologic and immunologic events in primary HIV infection. Springer Semin. Immunopathol. 18, 257–266 (1997).
Alter, G. & Altfeld, M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J. Intern. Med. 265, 29–42 (2009).
Alter, G. et al. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J. Infect. Dis. 195, 1452–1460 (2007).
Berger, C.T. & Alter, G. Natural killer cells in spontaneous control of HIV infection. Curr. Opin. HIV AIDS 6, 208–213 (2011).
Martin, M.P. et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31, 429–434 (2002).
Alter, G. et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 204, 3027–3036 (2007).
French, A.R. & Yokoyama, W.M. Natural killer cells and viral infections. Curr. Opin. Immunol. 15, 45–51 (2003).
Collins, K.L. & Baltimore, D. HIV's evasion of the cellular immune response. Immunol. Rev. 168, 65–74 (1999).
De Maria, A. et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur. J. Immunol. 33, 2410–2418 (2011).
Mavilio, D. et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl Acad. Sci. USA 102, 2886–2891 (2005).
Fogli, M. et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur. J. Immunol. 34, 2313–2321 (2004).
Cai, Q., Huang, X.L., Rappocciolo, G. & Rinaldo, C.R. Jr . Natural killer cell responses in homosexual men with early HIV infection. J. Acquir. Immune Defic. Syndr. 3, 669–676 (1990).
Poli, G. et al. Natural killer cells in intravenous drug abusers with lymphadenopathy syndrome. Clin. Exp. Immunol. 62, 128–135 (1985).
Vieillard, V., Fausther-Bovendo, H., Samri, A. & Debre, P. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J. Acquir. Immune Defic. Syndr. 53, 564–573 (2010).
Wilson, A.D., Stokes, C.R. & Bourne, F.J. Morphology and functional characteristics of isolated porcine intraepithelial lymphocytes. Immunology 59, 109–113 (1986).
Chai, J.Y. & Lillehoj, H.S. Isolation and functional characterization of chicken intestinal intra-epithelial lymphocytes showing natural killer cell activity against tumour target cells. Immunology 63, 111–117 (1988).
Flexman, J.P., Shellam, G.R. & Mayrhofer, G. Natural cytotoxicity, responsiveness to interferon and morphology of intra-epithelial lymphocytes from the small intestine of the rat. Immunology 48, 733–741 (1983).
Tagliabue, A., Befus, A.D., Clark, D.A. & Bienenstock, J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J. Exp. Med. 155, 1785–1796 (1982).
Chiba, M., Bartnik, W., ReMine, S.G., Thayer, W.R. & Shorter, R.G. Human colonic intraepithelial and lamina proprial lymphocytes: cytotoxicity in vitro and the potential effects of the isolation method on their functional properties. Gut 22, 177–186 (1981).
Eiras, P. et al. Intestinal intraepithelial lymphocytes contain a CD3- CD7+ subset expressing natural killer markers and a singular pattern of adhesion molecules. Scand. J. Immunol. 52, 1–6 (2000).
Leon, F. et al. Human small-intestinal epithelium contains functional natural killer lymphocytes. Gastroenterology 125, 345–356 (2003).
Carman, P.S. et al. Intraepithelial leukocytes contain a unique subpopulation of NK-like cytotoxic cells active in the defense of gut epithelium to enteric murine coronavirus. J. Immunol. 136, 1548–1553 (1986).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Crellin, N.K., Trifari, S., Kaplan, C.D., Cupedo, T. & Spits, H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J. Exp. Med. 207, 281–290 (2010).
Arnaud-Battandier, F., Bundy, B.M., O'Neill, M., Bienenstock, J. & Nelson, D.L. Cytotoxic activities of gut mucosal lymphoid cells in guinea pigs. J. Immunol. 121, 1059–1065 (1978).
Nauss, K.M., Pavlina, T.M., Kumar, V. & Newberne, P.M. Functional characteristics of lymphocytes isolated from the rat large intestine. Response to T-cell mitogens and natural killer cell activity. Gastroenterology 86, 468–475 (1984).
Tagliabue, A., Luini, W., Soldateschi, D. & Boraschi, D. Natural killer activity of gut mucosal lymphoid cells in mice. Eur. J. Immunol. 11, 919–922 (1981).
Moretta, A., Bottino, C., Mingari, M.C., Biassoni, R. & Moretta, L. What is a natural killer cell? Nat. Immunol. 3, 6–8 (2002).
Lopez-Verges, S. et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 116, 3865–3874 (2010).
Hamperl, H. & Hellweg, G. Granular endometrial stroma cells. Obstet. Gynecol. 11, 379–387 (1958).
Martin, M.P. et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39, 733–740 (2007).
Schacker, T.W. et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Invest. 110, 1133–1139 (2002).
Estes, J. et al. Collagen deposition limits immune reconstitution in the gut. J. Infect. Dis. 198, 456–464 (2008).
Veazey, R.S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003).
Mehandru, S. et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200, 761–770 (2004).
Estes, J.D. et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J. Infect. Dis. 195, 551–561 (2007).
Alter, G. et al. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J. Immunol. 178, 7658–7666 (2007).
Poggi, A. & Zocchi, M.R. HIV-1 Tat triggers TGF-beta production and NK cell apoptosis that is prevented by pertussis toxin B. Clin. Dev. Immunol. 13, 369–372 (2006).
Parrott, D.M. et al. Analysis of the effector functions of different populations of mucosal lymphocytes. Ann. NY Acad. Sci. 409, 307–320 (1983).
Hunt, P.W. et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197, 126–133 (2008).
Santamaria, M., Lopez-Beltran, A., Toro, M., Pena, J. & Molina, I.J. Specific monoclonal antibodies against leukocyte-restricted cell surface molecule CD43 react with nonhematopoietic tumor cells. Cancer Res. 56, 3526–3529 (1996).
Mendelson, M. et al. NKp46 O-glycan sequences that are involved in the interaction with hemagglutinin type 1 of influenza virus. J. Virol. 84, 3789–3797 (2010).
Acknowledgements
We thank Dr Joseph Misdraji for his expertise on our staining data, and Dr Georg Lauer and Michelle Tomlinson for their help with healthy control tissue collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Sips, M., Sciaranghella, G., Diefenbach, T. et al. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol 5, 30–40 (2012). https://doi.org/10.1038/mi.2011.40
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2011.40
This article is cited by
-
Gut Innate Immunity and HIV Pathogenesis
Current HIV/AIDS Reports (2021)
-
Effects of HIV infection and ART on phenotype and function of circulating monocytes, natural killer, and innate lymphoid cells
AIDS Research and Therapy (2018)
-
Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies
Mucosal Immunology (2016)
-
Human immunodeficiency virus and the gastrointestinal immune system: does highly active antiretroviral therapy restore gut immunity?
Mucosal Immunology (2012)