Abstract
The role of CD4+ T cells in bladder autoimmune inflammation has not been identified because of the lack of a proper animal model. We investigated CD4+ T-cell responses to bladder urothelial ovalbumin (OVA), a model self-antigen (Ag), in transgenic URO-OVA mice. The expression of bladder urothelial OVA rendered mice unresponsive to OVA and resulted in quick clearance of Ag-specific CD4+ T cells. Adoptive transfer of naive OVA-specific CD4+ T cells led to exogenous T-cell proliferation, activation, and bladder infiltration but no inflammatory induction. In contrast, adoptive transfer of preactivated OVA-specific CD4+ T cells induced bladder inflammation. Studies further demonstrated that CD4+ T cells induced bladder inflammation in URO-OVA mice depleted of CD8+ T cells or deficient in the recombinase activating gene-1 (Rag-1−/−). These results indicate that urothelial Ag-specific CD4+ T cells can function as direct effector cells to induce bladder autoimmune inflammation independent of CD8+ T cells.
Similar content being viewed by others
Introduction
The bladder mucosal immune system consists of an integrated network of tissues (urothelium, lamina propria, and detrusor), immune and nonimmune cells, and effector molecules of these cells. The function of this system is to protect the bladder from microbial infection. Like the intestinal and respiratory mucosal immune systems, the bladder mucosal immune system is highly immunoreactive and prone to inflammatory responses. In addition to bacterial-induced inflammation such as by Escherichia coli (a uropathogen) or Mycobacterium bovis bacillus Calmette-Guérin (an intravesical therapeutic agent), the bladder also develops noninfectious inflammation. For example, interstitial cystitis/painful bladder syndrome (IC/PBS) is a chronic inflammatory condition of the urinary bladder characterized by pelvic pain, irritative voiding symptoms (frequency, urgency, and nocturia), and sterile and cytologically normal urine.1, 2 Although the etiology of IC/PBS remains unknown, the immune/autoimmune mechanisms are thought to have at least a partial role in the pathophysiology of this painful condition.3, 4, 5, 6, 7, 8
The mechanisms of autoimmune inflammation are complex and multifactorial. However, T-cell acquisition of autoreactivity is common in numerous human and experimental autoimmune diseases.9, 10, 11, 12, 13, 14 Involvement of both CD4+ and CD8+ T cells has been observed in the majority of T cell-mediated autoimmune diseases. However, one T-cell subset may have a predominant role over the other in a defined autoimmune disease. Under normal conditions, the bladder mucosa contains few T cells representing homeostasis.15, 16 CD8+ T cells are sparsely scattered within the urothelium, whereas CD8+ T cells and to a lesser extent CD4+ T cells are present in the lamina propria.15 However, in IC/PBS the number of T cells in the bladder increases with CD4+ T cells being predominant over CD8+ T cells.15, 16 These observations suggest that CD4+ T cells are preferentially induced in IC/PBS.7, 15, 16, 17, 18 However, despite these observations, little is known about the role of CD4+ T cells in bladder autoimmune inflammation. It is generally accepted that after activation in lymphoid tissues, autoreactive CD4+ T cells migrate to target organ(s) that express corresponding self-antigen (Ag) and cause inflammation in the affected organ(s).19, 20, 21, 22, 23 Accordingly, certain molecules reflecting the effector status of CD4+ T cells, such as interferon (IFN)-γ, perforin, and Fas ligand, can be detected in the inflammatory site(s).24, 25, 26
Previous studies on bladder autoimmune inflammation were based on the use of bladder tissue homogenate as an immunogen. Although this conventional method has been actively used in IC/PBS research and provided a useful tool for investigation of bladder autoimmune inflammation,27, 28, 29, 30, 31 this method does not facilitate the detailed mechanistic studies with regard to autoreactive T-cell responses because of its lack of defined self-Ag and its corresponding T-cell receptor (TCR) specificity. To cope with the drawbacks of this method, we recently developed a transgenic model of bladder autoimmune inflammation, designated as URO-OVA mice.32 URO-OVA mice express a membrane form of the model Ag OVA as a self-Ag on the bladder urothelium and develop bladder inflammation upon introduction of Ag-specific CD8+ T cells.32 In addition, the expression of bladder urothelial OVA leads to Ag-specific CD8+ T-cell tolerance, activation, and autoimmune responses in these mice.32 In this study we extended to investigate CD4+ T-cell responses in URO-OVA mice. We observed that the expression of bladder urothelial OVA rendered mice unresponsive to OVA and resulted in quick clearance of Ag-specific CD4+ T cells (despite they gained proliferation and activation). We further observed that URO-OVA mice developed bladder autoimmune inflammation after transfer of preactivated Ag-specific CD4+ T cells. Importantly, by using URO-OVA mice depleted of CD8+ T cells or deficient in Rag-1 (recombinase activating gene-1), we observed that urothelial Ag-specific CD4+T cells functioned as direct effector cells and induced bladder autoimmune inflammation independent of CD8+ T cells.
Results
Expression of bladder urothelial OVA renders mice unresponsive to OVA and results in quick clearance of OVA-specific CD4+ T cells
To determine the impact of the expression of bladder urothelial OVA on host immune responses to OVA Ag, we immunized URO-OVA mice with OVA323–339 peptide emulsified with complete Freund's adjuvant. Sex- and age-matched C57BL/6 (B6) mice were immunized as a control. After 14 days, splenocytes were prepared, restimulated with OVA323–339 peptide in vitro, and analyzed for IFN-γ production by enzyme-linked immunosorbent assay (Figure 1). Compared with B6 splenocytes, splenocytes from URO-OVA mice produced no IFN-γ in response to OVA323–339 peptide restimulation, although both splenocytes produced a similar level of IFN-γ in response to phorbol myristate acetate and ionomycin, the two known positive T-cell stimulators. We further challenged both OVA-immunized URO-OVA and B6 mice intravesically with OVA323–339 peptide at day 14 and found that only B6 mice, but not URO-OVA mice, developed histological bladder inflammation (data not shown). These observations suggested that constitutive expression of bladder urothelial OVA rendered mice unresponsive to OVA Ag.
URO-OVA mice show no responsiveness to ovalbumin (OVA) stimulation. Both URO-OVA mice and B6 mice were immunized by subcutaneous (SC) injection of 100 μg of OVA323–339 peptide emulsified with complete Freund's adjuvant (CFA) at day 0 and killed for analysis at day 14. Splenocytes were prepared and cultured in the absence or presence of phorbol myristate acetate (PMA) plus ionomycin (PI; 100 and 1,500 ng ml–1, respectively), a control ras peptide (CP; 10 μg ml–1), or OVA323–339 peptide (10 μg ml–1) for 3 days. The production of interferon-γ (IFN-γ) in culture supernatants was then analyzed by enzyme-linked immunosorbent assay (ELISA) and presented as mean±s.d. from triplicate determinations. *P<0.01 by Student's t-test.
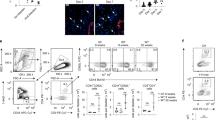
To determine whether the expression of bladder urothelial OVA could affect host tolerance to self-Ag-specific CD4+ T cells, we adoptively transferred URO-OVA mice (Thy1.2) with naive splenocytes from OT-II mice (Thy1.1), a transgenic line that expressed CD4 TCR (Vα2Vβ5) specific for I-Ab/OVA323–339 epitope.33, 34 Mice were killed for analysis at 1, 4, and 7 days after cell transfer. B6 mice (Thy1.2) were included for comparison. Flow cytometry was used to analyze exogenous OT-II CD4+Thy1.1+ T cells in the spleens and bladder-draining lymph nodes (BLNs; Figure 2a and b). In B6 mice, the highest number of CD4+Thy1.1+ T cells was observed at day 1. Then, the number of CD4+Thy1.1+ T cells returned to a baseline level at days 4 and 7. In contrast to B6 mice, in URO-OVA mice the number of CD4+Thy1.1+ T cells was low (baseline levels) at day 1, peaked at day 4 (20-fold increase in the spleens and 10-fold increase in the BLNs), and returned to the baseline levels at day 7. The observed increased numbers of CD4+Thy1.1+ T cells in the spleens and BLNs of URO-OVA mice suggested that the bladder urothelial OVA was antigenic and could access the immune system for CD4+ T-cell stimulation. However, the numbers of CD4+Thy1.1+ T cells at day 1 was fivefold less in URO-OVA mice than in B6 mice, suggesting that these urothelial Ag-specific CD4+ T cells encountered host clearance within 24 h after transfer in the former mice. The quick decrease in CD4+Thy1.1+ T-cell numbers from day 4 to day 7 in URO-OVA mice further suggested the ability of these mice to quick clear self-Ag-specific CD4+ T cells. The CD4+Thy1.1+ T cells became less in URO-OVA mice than in B6 mice at day 14 (0.06 vs. 0.18% in the spleens and 0.07 vs. 0.17% in the BLNs) and negligible in both mice at day 21 and thereafter.
URO-OVA mice exhibit quick clearance of adoptively transferred ovalbumin (OVA)-specific CD4+ T cells. OT-II splenocytes (Thy1.1) were prepared and transferred intravenously (IV) into URO-OVA mice and B6 mice (2 × 107 cells per mouse; both Thy1.2 background) at day 0. Mice were killed for analysis at days 1, 4, and 7. The spleens and bladder-draining lymph nodes (BLNs) were collected and analyzed for CD4+Thy1.1+ T cells by flow cytometry. A gate was set on lymphocytes according to scatter criteria. The percentage of CD4+Thy1.1+ T cells in total lymphocytes is indicated. (a) A representative flow cytometric analysis for URO-OVA mice vs. B6 mice. (b) The kinetics of clearance of OT-II CD4+Thy1.1+ T cells in URO-OVA mice vs. B6 mice; n=6–10 mice for each time point.
Adoptively transferred naive OVA-specific CD4+ T cells gain proliferation, activation, and bladder infiltration in URO-OVA mice
To further assess Ag-specific CD4+ T-cell responses to bladder urothelial OVA, we adoptively transferred URO-OVA mice with naive OT-II splenocytes labeled with carboxyfluoroscein succinimidyl ester, followed by flow cytometric analysis of transferred OT-II CD4+ T cells. B6 mice were included for comparison. At day 4 after cell transfer, a clear T-cell division was observed in the BLNs (49.3%) of URO-OVA mice but not in the BLNs of B6 mice (Figure 3a). In addition, the bladders of the same URO-OVA mice also showed increased carboxyfluoroscein succinimidyl ester-positive infiltrating cells compared with the bladders of B6 mice (1,257 cells vs. 87 cells; Figure 3b). These observations supported the antigenicity of the bladder urothelial OVA and its accessibility to the immune system for CD4+ T-cell stimulation. Also, as OT-II CD4+ T cells accumulated more in the bladders of URO-OVA mice than in the bladders of B6 mice, it appeared that the bladder urothelial OVA served as a target for OVA-specific CD4+ T cells.
Naive ovalbumin (OVA)-specific CD4+ T cells gain proliferation, activation, and homing to the bladder in URO-OVA mice. (a) CD4+ T-cell proliferation in URO-OVA mice. OT-II splenocytes were prepared, labeled with carboxyfluoroscein succinimidyl ester (CFSE), and transferred intravenously (IV) into URO-OVA mice and B6 mice (2 × 107 cells per mouse) at day 0. The bladder-draining lymph nodes (BLNs) were collected at day 4 and analyzed for CFSE-positive cells by flow cytometry. A gate was set on CD4+ T cells. A representative of three mice for each group is shown. (b) CD4+ T-cell homing to the bladders of URO-OVA mice. The bladders of the same mice were collected at day 4. Single-cell suspensions were prepared and analyzed for CFSE-positive cells by flow cytometry. Total CFSE-positive cells in each bladder are indicated. A representative of three bladders for each group is shown. (c) CD4+ T-cell activation in URO-OVA mice. OT-II splenocytes (Thy1.1) were prepared and transferred IV into URO-OVA mice and B6 mice (2 × 107 cells per mouse; both Thy1.2 background) at day 0. The BLNs were collected at day 4 and analyzed for CD44, CD45RB, CD62L, and CD69 by flow cytometry. A gate was set on CD4+Thy1.1+ T cells. A representative of three mice for each group is shown. Hollow curve: B6 mice; filled curve: URO-OVA mice.
In a separate experiment, both URO-OVA mice (Thy1.2) and B6 mice (Thy1.2) were transferred intravenously (IV) with naive OT-II splenocytes (Thy1.1) and analyzed 4 days later for the activation status of transferred OT-II CD4+ T cells by flow cytometry. Compared with B6 mice, CD4+Thy1.1+ T cells from the BLNs of URO-OVA mice expressed increased CD44 and decreased CD45RB and CD62L (Figure 3c). There was no change for CD69 in the BLNs of URO-OVA mice, presumably because of CD69 being an early activation marker.35 However, despite these T-cell proliferation, activation, and bladder infiltration, adoptive transfer of naive OVA-specific CD4+ T cells induced no bladder histopathology in URO-OVA mice.
Adoptive transfer of preactivated OVA-specific CD4+ T cells induces bladder autoimmune inflammation independent of CD8+ T cells in URO-OVA mice
We next tested whether OVA-specific CD4+ T cells upon activation could induce bladder inflammation in URO-OVA mice. To test this, OT-II CD4+ T cells were prepared, activated in vitro with OVA323–339 peptide, and transferred IV into URO-OVA mice for cystitis induction at day 0. Mice were killed for analysis at day 10. The in vitro activation of OT-II CD4+ T cells increased the clonal phenotype Vβ5-positive CD4+ T cells from 38 to 92% (data not shown). To further test whether CD4+ T cells had an ability to induce bladder inflammation in the absence of CD8+ T cells, URO-OVA mice were injected intraperitoneally with a CD8+ T cell-depleting antibody (clone: 2.43) in 3-day intervals from days −3 to 9. Mice were transferred IV with preactivated OT-II CD4+ T cells for cystitis induction at day 0 and killed for analysis at day 10. Flow cytometry showed nearly complete CD8+ T-cell depletion in both spleen (1 vs. 10%) and BLNs (2 vs. 25%) at day 10 (Figure 4a). However, despite this CD8+ T-cell depletion, mice developed histological bladder inflammation (Figure 4b, right panel; score: 2+) although its severity was less than that of mice without CD8+ T-cell depletion (Figure 4b, middle panel; score: 2 to 3+). Both inflamed bladders of 2.43-treated and nontreated mice showed clear edema and cellular infiltration by histological hematoxylin and eosin staining. In addition, the inflamed bladders of 2.43-treated mice also produced increased neuroinflammatory factor mRNAs such as tumor necrosis factor-α, nerve growth factor, and substance P precursor (Figure 4c), although the levels of mRNAs produced was 20–30% lower than those of the bladders of nontreated mice. Nevertheless, these observations indicated that urothelial Ag-specific CD4+ T cells could function as direct effector cells to induce bladder autoimmune inflammation independent of CD8+ T cells.
Preactivated ovalbumin (OVA)-specific CD4+ T cells induce bladder inflammation independent of CD8+ T cells in URO-OVA mice. URO-OVA mice were injected intraperitoneally (IP) with 2.43 antibody for CD8+ T-cell depletion in 3-day intervals from day −3 to day 9. OT-II CD4+ T cells were prepared, activated with OVA323–339 peptide in vitro, and transferred intravenously (IV) into URO-OVA mice (1 × 107 cells per mouse) for cystitis induction at day 0. Mice were killed for analysis at day 10. (a) Flow cytometric analysis of CD8+ T-cell depletion in the spleen (left panel) and bladder-draining lymph nodes (BLNs; right panel) of URO-OVA mice treated with 2.43 antibody. URO-OVA mice without 2.43 treatment were included for comparison. A gate was set on lymphocytes according to scatter criteria. The percentage of both CD4+ and CD8+ T-cell subsets in total lymphocytes is indicated. (b) Histological hematoxylin and eosin (H&E) staining of the bladders of URO-OVA mice treated with (right panel) or without (middle panel) 2.43 antibody at day 10. The normal bladders of URO-OVA mice (left panel) are included for comparison. Cellular infiltration and edema are indicated by green and red arrows, respectively. A representative of three bladders for each group is shown. (c) Reverse transcriptase-PCR (RT-PCR) analysis of tumor necrosis factor-α (TNF-α), nerve growth factor (NGF), and substance P precursor (pre-SP) mRNAs in the bladders of URO-OVA mice treated with or without 2.43 antibody at day 10. Specific band intensity is presented after normalization with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). 1 and 2: the normal bladders; 3 and 4: the inflamed bladders of mice with no 2.43 treatment; 5 and 6: the inflamed bladders of mice treated with 2.43 antibodies.
Adoptive transfer of preactivated OVA-specific CD4+ T cells induces bladder autoimmune inflammation in URO-OVA/Rag-1−/− mice
To verify the effector role of OVA-specific CD4+ T cells, we generated URO-OVA/Rag-1−/− mice, a crossed line of URO-OVA mice and Rag-1−/− mice, that retained the bladder urothelial OVA expression but lacked endogenous T cells. Both URO-OVA (Thy1.2) and URO-OVA/Rag-1−/− mice (Thy1.2) were transferred IV with preactivated OT-II CD4+ T cells (Thy1.1) for cystitis induction at day 0 and killed for analysis at day 10. Despite the lack of endogenous T cells, URO-OVA/Rag-1−/− mice developed histological bladder inflammation with severer cellular infiltration (Figure 5a, right panel) than URO-OVA mice (Figure 5a, middle panel). Flow cytometric analysis of bladder-infiltrating T cells showed the presence of both CD4+ (Figure 5b) and Thy1.1+ T cells (Figure 5c) in the inflamed bladders. However, in the bladders of URO-OVA/Rag-1−/− mice, the number of CD4+ T cells matched the number of Thy1.1+ T cells (48,220 cells vs. 48,104 cells), suggesting that they were all transferred exogenous OT-II CD4+Thy1.1+ T cells. In contrast, the bladders of URO-OVA mice contained more CD4+ T cells than Thy1.1+ cells (21,236 cells vs. 16,012 cells), suggesting that endogenous CD4+ T cells were also recruited during bladder inflammatory responses. Consistent with histological hematoxylin and eosin staining, flow cytometry showed twofold higher CD4+ T cells (48,220 cells vs. 21,236 cells) and threefold higher Thy1.1+ cells (48,104 cells vs. 16,012 cells) in the bladders of URO-OVA/Rag-1−/− mice than in the bladders of URO-OVA mice. In addition, similar to the inflamed bladders of URO-OVA mice, the inflamed bladders of URO-OVA/Rag-1−/− mice expressed increased mRNAs of T-cell effector molecules IFN-γ, perforin, and Fas ligand (Figure 5d). Immunohistochemistry analysis further revealed the expression of OVA and I-Ab on the bladder urothelium of URO-OVA/Rag-1−/− mice that received preactivated OT-II CD4+ T cells (Figure 6; OVA not shown). Numerous T cells were found in the urothelial layer other than submucosal regions, suggesting that OVA-specific CD4+ T cells interacted with I-Ab/OVA323–339-expressing urothelial cells. These observations supported that urothelial Ag-specific CD4+ T cells could function as direct effector cells and induce bladder autoimmune inflammation without CD8+ T cells.
Preactivated ovalbumin (OVA)-specific CD4+ T cells induce bladder inflammation in URO-OVA/Rag-1−/− mice. OT-II CD4+ T cells (Thy1.1) were prepared, activated with OVA323–339 peptide in vitro, and transferred intravenously (IV) into URO-OVA/Rag-1−/− mice (1 × 107 cells per mouse) for cystitis induction at day 0. Mice were killed for analysis at day 10. URO-OVA mice were included for comparison. (a) Histological hematoxylin and eosin (H&E) staining of the bladders of URO-OVA mice (middle panel) and URO-OVA/Rag-1−/− mice (right panel) at day 10. The normal bladders of URO-OVA/Rag-1−/− mice (left panel) are included for comparison. Cellular infiltration and edema are indicated by green and red arrows, respectively. A representative of 5 to 6 bladders for each group is shown. (b, c) Flow cytometric analysis of bladder-infiltrating CD4+ and Thy1.1+ T cells in the bladders of URO-OVA mice (middle panel) and URO-OVA/Rag-1−/− mice (right panel) at day 10. The normal bladders of URO-OVA/Rag-1−/− mice (left panel) are included for comparison. A gate was set on lymphocytes according to scatter criteria. Total infiltrating CD4+ and Thy1.1+ T cells in each bladder are indicated. A representative of 5 to 6 bladders for each group is shown. (d) Reverse transcriptase-PCR (RT-PCR) analysis of interferon-γ (IFN-γ), perforin, and Fas ligand (FasL) mRNAs in the bladders of URO-OVA mice and URO-OVA/Rag-1−/− mice at day 10. Mean specific band intensity is presented after normalization with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). URO/Rag-1−/−: URO-OVA/Rag-1−/− mice.
Ovalbumin (OVA)-specific CD4+ T cells infiltrate into the bladder urothelium of URO-OVA/Rag-1−/− mice. The bladders of URO-OVA/Rag-1−/− mice were collected 10 days after adoptive transfer of preactivated OT-II CD4+ T cells (1 × 107 cells per mouse). Bladder sections were prepared and analyzed by immunohistochemistry (IHC) using (a) control IgG2a or (b–d) anti-mouse I-Ab antibody (Ab). Brown staining indicates the urothelial cell expression of I-Ab. Lymphocytes in the urothelium and submucosa are indicated by arrows. MHC, major histocompatibility complex.
Discussion
Research in autoimmune mechanisms of the bladder mucosal immune system has been hindered because of the lack of a proper animal model. A few scattered studies suggested that CD4+ T cells were preferentially induced over CD8+ T cells in IC/PBS.7, 15, 16, 17, 18 However, the actual role of CD4+ T cells in bladder autoimmune inflammation has not been determined. In this study we used our recently developed transgenic URO-OVA mice that express the model Ag OVA on the bladder urothelium to delineate the role of CD4+ T cells in bladder autoimmunity. We have observed that CD4+ T cells actively respond to the bladder urothelial OVA and function as direct effector cells to induce bladder autoimmune inflammation independent of CD8+ T cells.
Constitutive expression of OVA on the bladder urothelium renders CD4+ T cells tolerance to OVA stimulation in URO-OVA mice, as mice immunized with OVA323–339 peptide failed to develop specific immune responses (Figure 1). Similarly, URO-OVA mice developed no OVA-specific immune response after immunization with Ad-OVA (data not shown), a OVA-delivering recombinant adenovirus.36 In addition, URO-OVA mice also exhibited early and quick clearance of adoptively transferred OVA-specific CD4+ T cells (Figure 2). However, despite this host tolerance and clearance, adoptively transferred naive OVA-specific CD4+ T cells gained proliferation and activation in the BLNs and homing to the bladders in URO-OVA mice (Figure 3). These observations differed from those in other studies in which adoptively transferred naive OVA-specific CD4+ T cells failed to infiltrate into target organs such as the pancreas in RIP-mOVA mice (a transgenic line expressing OVA in pancreatic islets),37 the brain and spinal cord in ODC-OVA mice (a transgenic line expressing OVA in oligodendrocytes),38, 39 and the liver in TF-OVA mice (a transgenic line expressing OVA in hepatocytes) and ASRT-OVA mice (a transgenic line expressing OVA in cholangiocytes).40 One possible explanation for this discrepancy could be because of the use of different tissue-specific promoters that drive the different levels of transgenic OVA expression in target organs. Alternatively, the different expression levels of major histocompatibility complex class II molecules in target organs could affect CD4+ T-cell recognition of transgenic OVA Ag expressed in these organs. Despite naive OVA-specific CD4+ T cells could gain proliferation, activation, and bladder infiltration in URO-OVA mice (Figure 3), they failed to induce bladder histopathology, presumably because of the host quick clearance of the autoreactive T cells. Nevertheless, our observations suggest that the bladder urothelial OVA could access the immune system for CD4+ T-cell stimulation and serve as a target for CD4+ T-cell bladder infiltration. However, how antigen-presenting cells acquire urothelial OVA in the bladder, present it to CD4+ T cells in the BLNs, and cause CD4+ T-cell migration from the BLNs to the bladder remain to be determined.
In contrast to naive OVA-specific CD4+ T cells, adoptive transfer of preactivated OVA-specific CD4+ T cells induced histological bladder inflammation in URO-OVA mice (Figure 4b, middle panel). This observation was consistent with those in other studies that adoptive transfer of preactivated OVA-specific CD4+ T cells caused β-cell destruction and a rapid onset of diabetes in RIP-mOVA mice.41 Analysis of bladder-infiltrating T cells showed CD4+ T cells to be predominant over CD8+ T cells (data not shown). Strikingly, studies demonstrated that CD4+ T cells could even induce histological bladder inflammation independent of CD8+ T cells in URO-OVA mice (Figure 4b, right panel). The severity of bladder inflammation in CD8+ T cell-depleted mice was comparable with mice without CD8+ T-cell depletion, although the former mice exhibited slightly reduced cellular infiltration in the bladders (likely because of the depletion of endogenous CD8+ T cells). In addition, the inflamed bladders of CD8+ T cell-depleted mice produced increased neuroinflammatory factor mRNAs (i.e., tumor necrosis factor-α, nerve growth factor, and substance P precursor; Figure 4c), similar to those of nondepleted mice, although the former bladders produced less mRNAs than the latter bladders. The effector role of CD4+ T cells in the induction of bladder inflammation was further verified in URO-OVA/Rag-1−/− mice that retained the bladder urothelial OVA expression but lacked endogenous CD4+ and CD8+ T cells. Compared with URO-OVA mice (Figure 5a, middle panel), URO-OVA/Rag-1−/− mice developed similar histological bladder inflammation (Figure 5a, right panel). In addition, adoptively transferred OVA-specific CD4+ T cells were observed to infiltrate into the bladder urothelium other than the submucosal regions in URO-OVA/Rag-1−/− mice (Figure 6). These observations indicate that CD4+ T cells are capable of functioning as direct effector cells to induce bladder autoimmune inflammation independent of CD8+ T cells.
Analysis of bladder-infiltrating T cells showed exogenous CD4+Thy1.1+ T cells to be threefold more in URO-OVA/Rag-1−/− mice than in URO-OVA mice (Figure 5c). This increased CD4+Thy1.1+ T-cell infiltration presumably resulted from the compensatory activity of these cells because of the lack of endogenous T cells in URO-OVA/Rag-1−/− mice. To support this assumption, the similar increase in CD4+ T-cell population and activity has previously been observed in CD8+ T cell-deficient mice.42, 43 Also, the inflamed bladders of URO-OVA mice were found to contain more CD4+ T cells (Figure 5b, middle panel) than Thy.1.1+ T cells (Figure 5c, middle panel), suggesting that endogenous CD4+ T cells actively involved in the development of bladder autoimmune inflammation. In addition, endogenous CD8+ T cells were also found in the inflamed bladders of URO-OVA mice (data not shown). Thus, it appears that multiple T-cell clones with different Ag specificities could be elicited during bladder autoimmune process. These OVA-nonspecific T cells might be specific for the as yet unknown self-Ag(s) in the bladder and recruited by epitope spreading, a phenomenon known in some autoimmune diseases and models.44, 45, 46, 47, 48 The investigation on the role of endogenous T cells in bladder autoimmune inflammation is currently underway.
To further determine the effector role of CD4+ T cells, we analyzed mRNA expression of three common T-cell effector molecules, IFN-γ,24 perforin,25 and Fas ligand,26 in the inflamed bladders of URO-OVA mice and URO-OVA/Rag-1−/− mice. We observed that the inflamed bladders of both mice expressed similar mRNA levels of the effector molecules (Figure 5d). As URO-OVA/Rag-1−/− mice lacked endogenous T cells, the source of the effector molecules was the adoptively transferred OVA-specific CD4+ T cells. This observation, together with the bladder histopathology observed in CD8+ T cell-depleted URO-OVA mice and URO-OVA/Rag-1−/− mice, supports the effector role of CD4+ T cells in the development of bladder autoimmune inflammation. The observation that the inflamed bladders of URO-OVA mice expressed similar levels of effector molecule mRNAs further supports the contribution of endogenous CD4+ and CD8+ T cells to bladder autoimmune inflammation in these mice.
In summary, we have demonstrated that CD4+ T cells are responsive to bladder urothelial Ag and function as direct effector cells in the development of bladder autoimmune inflammation. These results, together with those from our previous studies,32, 49 shed light on autoreactive T-cell responses in bladder autoimmunity. A clear understanding of bladder autoimmune process will add to the development of effective interventions for bladder inflammatory diseases that contain immune/autoimmune mechanisms in the pathophysiology such as IC/PBS.
Methods
Mice. URO-OVA mice (Thy1.2 congenic; B6 background) were developed previously in our laboratory.32 B6 mice were obtained from National Cancer Institute/Frederick Cancer Research Animal Facility (Frederick, MD). OT-II mice, a line originally generated by Barnden et al.33, 34 that expresses the transgenic CD4 TCR (Vα2+Vβ5+) specific for the I-Ab/OVA323–339 epitope, were maintained on the Thy1.1 background. Certain URO-OVA mice were also maintained on the Rag-1−/− background. Mice were used at 6–8 weeks. Male OT-II mice were used because only the Y chromosome carried the OVA-specific CD4 TCR transgene. All mice were housed in a pathogen-free facility at the University of Iowa Animal Care Facility and used according to the procedures approved by University of Iowa animal care and use committee.
Analysis of immune responsiveness to OVA. Mice were immunized by subcutaneous injection of 100 μg of OVA323–339 peptide emulsified with complete Freund's adjuvant (Sigma-Aldrich, St Louis, MO) and killed for analysis 14 days later. Splenocytes were prepared and cultured in the absence or presence of phorbol myristate acetate (100 ng ml–1) plus ionomycin (1,500 ng ml–1), a control ras peptide (10 μg ml–1), or OVA323–339 peptide (10 μg ml–1) for 3 days at 37 °C in a humidified incubator with 5% CO2. The production of IFN-γ in culture supernatants was then evaluated using enzyme-linked immunosorbent assay with appropriate paired antibodies (clones: R4.6A2 and XMG1.2; Endogen, Woburn, MA) according to the manufacturer's instruction.
Analysis of CD4+ T-cell induction in vivo. Splenocytes of OT-II mice were prepared by mechanical disruption of the spleen, followed by ACK (ammonium chloride lysing buffer) lysis as described previously.32 Cells (2 × 107 cells per mouse) were transferred IV into URO-OVA mice and control B6 mice. At the indicated time points, the spleen, BLNs, and bladder were collected. Single-cell suspensions were prepared, stained with antibodies (eBioscience, San Diego, CA) to CD4 (clone: RM4-5), CD44 (clone: IM7), CD45RB (clone: C363.16A), CD62L (clone: MEL-14), CD69 (clone: H1.2F3), or Thy1.1 (clone: HIS51), and analyzed by flow cytometry. For some experiments, OT-II splenocytes were labeled with 5 μM of carboxyfluoroscein succinimidyl ester (Molecular Probes, Carlsbad, CA) and transferred IV into URO-OVA mice and B6 mice (2 × 107 cells per mouse) as described previously.32 After 4 days, the spleen, BLNs, and bladder were collected. Single-cell suspensions were prepared and analyzed for carboxyfluoroscein succinimidyl ester-positive cells by flow cytometry.
Induction of bladder inflammation. OVA-specific CD4+ T cells were prepared from splenocytes of OT-II mice using the MACS magnetic isolation system (Miltenyi Biotec, San Francisco, CA) and CD4 (L3T4) microbeads according to the manufacturer's instruction. The purity of the isolated CD4+ T cells was 95%. Cells were activated in vitro with OVA323–339 peptide (10 μg ml–1) in the presence of syngeneic D1 dendritic cells for 3 days at 37 °C in a humidified incubator with 5% CO2.50 After Ficoll-Pague gradient centrifugation, living cells were collected, counted, and transferred IV into URO-OVA mice (1 × 107 cells per mouse) for cystitis induction. Mice were killed 10 days after cell transfer for analysis. To determine whether CD4+ T cells could induce bladder inflammation independent of CD8+ T cells, URO-OVA mice were injected intraperitoneally with 250 μg per dose of a CD8-depleting antibody (clone: 2.43) in 3-day intervals starting at day −3 up to day 9. Mice were transferred IV with preactivated OT-II CD4+ T cells for cystitis induction at day 0 and killed for analysis at day 10. To confirm the effector role of CD4+ T cells, URO-OVA/Rag-1−/− mice were transferred IV with preactivated OT-II CD4+ T cells (1 × 107 cells per mouse) for cystitis induction at day 0 and killed for analysis at day 10. The bladders were collected and analyzed for bladder inflammation.
Bladder histological analysis. The standard paraffin-embedded histological sections of the bladder were prepared and stained with hematoxylin and eosin solution as described previously.32, 49 Bladder inflammation was scored based on cellular infiltration in the lamina propria and interstitial edema as follows: 1+ (mild infiltration with no or mild edema); 2+ (moderate infiltration with moderate edema); and 3+ (moderate to severe infiltration with severe edema).32, 49
The bladder I-Ab immunohistochemistry was performed as described previously.32 Briefly, the bladders were fixed in 2% buffered formalin, embedded in paraffin, and cut into 8 μm sections. After Ag retrieval and blocking, the slides were incubated with biotinylated anti-mouse I-Ab antibody (clone: AF6-120.1; BD Biosciences Pharmingen, San Diego, CA) or control mouse IgG2a (clone: MOPC-173) for overnight, followed by development with horseradish peroxidase-labeled streptavidin and diaminobenzidine substrate solution. After rinsing, the slides were counterstained with hematoxylin and eosin solution and viewed with an Olympus microscope, .
Flow cytometric analysis of T-cell responses. In various experiments, single-cell suspensions of the spleen, BLNs, and bladder were prepared as described previously.32, 49 Cells were washed with staining buffer (1% fetal bovine serum, 0.09% (w/v) NaN3 in Mg2+- and Ca2+-free phosphate-buffered saline), stained with fluorescein isothiocyanate-, phycoerythrin-, or phycoerythrin–Cy5-labeled antibody (eBioscience) to various surface markers (including CD4, CD44, CD45RB, CD62L, CD69, Thy1.1, and OT-II CD4 TCR clonal phenotype Vα2 or Vβ5) at 4 °C for 15 min, fixed in 2% formalin, and analyzed using a FACScan equipped with CellQuest (BD Biosciences, San Jose, CA). Postacquisition analysis was carried out using FlowJo software (Tree Star, Ashland, OR).
Reverse transcriptase-PCR analysis of neuroinflammatory factors and T-cell effector molecules. Total RNAs of the bladder were extracted using Qiagen RNAeasy Kit (Qiagen, Valencia, CA). Total RNAs (3 μg) were used for complementary DNA synthesis using Invitrogen Superscript III RNase H reverse transcriptase (Carlsbad, CA) and oligo dT according to the manufacturer's instruction. Complementary DNA products (2 μl) were further processed for PCR amplification using sequence-specific primer pairs and Invitrogen Taq DNA polymerase. The following primer pairs were used: 5′-CGTCAGCCGATTTGCTATCT-3′ and 5′-CGGACTCCGCAAAGTCTAAG-3′ for tumor necrosis factor-α (206 bp); 5′-CTGTGGACCCCAGACTGTTT-3′ and 5′-CACTGAGAACTCCCCCATGT-3′ for nerve growth factor (194 bp); 5′-GCCAATGCAGAACTACGAAA-3′ and 5′-GCTTGGACAGCTCCTTCATC-3′ for substance P precursor (280 bp); 5′-TGAACGCTACACACTGCATCT-3′ and 5′-GACTCCTTTTCCGCTTCCTGA-3′ for IFN-γ (459 bp); 5′-AGGTCTCCCCACTCTGGTTT-3′ and 5′-GTAGCACACGCCTTTAATCC-3′ for perforin (233 bp); 5′-CATCACAACCACTCCCACTG-3′ and 5′-GTTCTGCCAGTTCCTTCTGC-3′ for Fas ligand (162 bp); and 5′-GTTCCAGTATGACTCCACT-3′ and 5′-GTGCAGGATGCATTGCTG-3′ for GAPDH (glyceraldehyde-3-phosphate dehydrogenase; 321 bp). PCR kinetics was initially established to achieve desirable discrepancies between the normal bladders and the inflamed bladders. Based on the kinetics, 20 cycles were used for GAPDH, 32 cycles were used for IFN-γ, and 29 cycles were used for other molecules. The DNA fragments were run on a 1% agarose gel, stained with ethidium bromide, imaged by the EpiChemi digital image system (Upland, CA), and analyzed using Bio-Rad Quantity One software (Hercules, CA).
References
Warren, J.W., Brown, J., Tracy, J.K., Langenberg, P., Wesseimann, U. & Greenberg, P. Evidence-based criteria for pain of interstitial cystitis/painful bladder syndrome in women. Urology 71, 444–448 (2008).
Selo-Ojeme, D.O. & Onwude, J.L. Interstitial cystitis. J. Obstet. Gynaecol. 24, 216–225 (2004).
Van de Merwe, J.P. Interstitial cystitis and systemic autoimmune diseases. Nat. Clin. Prac. Urol. 4, 484–491 (2007).
Peeker, R., Atanasiu, L. & Logadottir, Y. Intercurrent autoimmune conditions in classic and non-ulcer interstitial cystitis. Scand. J. Urol. Nephrol. 37, 60–63 (2003).
Van de Merwe, J.P., Yamada, T. & Sakamoto, Y. Systemic aspects of interstitial cystitis, immunology and linkage with autoimmune disorders. Int. J. Urol. 10 (Suppl), S35–S38 (2003).
Ochs, R.L. Autoantibodies and interstitial cystitis. Clin. Lab. Med. 17, 571–579 (1997).
Ratliff, T.L., Klutke, C.G., Hofmeister, M., He, F., Russell, J.H. & Becich, M.J. Role of the immune response in interstitial cystitis. Clin. Immunol. Immunopathol. 74, 209–216 (1995).
Christmas, T.J. & Bottazzo, G.F. Abnormal urothelial HLA-DR expression in interstitial cystitis. Clin. Exp. Immunol. 87, 450–454 (1992).
Bahbouhi, B. et al. T cell recognition of self-antigen presenting cells by protein transfer assay reveals a high frequency of anti-myelin T cells in multiple sclerosis. Brain 133 (Pt 6), 1622–1636 (2010).
Okajima, M. et al. Analysis of T cell receptor Vbeta diversity in peripheral CD4 and CD8 T lymphocytes in patients with autoimmune thyroid diseases. Clin. Exp. Immunol. 155, 166–172 (2009).
Francis, J.N., Sabroe, I., Lloyd, C.M., Durham, S.R. & Till, S.J. Elevated CCR6+ CD4+ T lymphocytes in tissue compared with blood and induction of CCL20 during the asthmatic late response. Clin. Exp. Immunol. 152, 440–447 (2008).
Alaibac, M. et al. Role of cellular immunity in the pathogenesis of autoimmune skin diseases. Clin. Exp. Rheumatol. 24 (1 Suppl 40), S14–S19 (2006).
Hayward, S.L., Bautista-Lopez, N., Suzuki, K., Atrazhev, A., Dickie, P. & Elliott, J.F. CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice. J. Immunol. 176, 7715–7725 (2006).
Amend, B. et al. Induction of autoimmunity by expansion of autoreactive CD4+CD62Llow cells in vivo. J. Immunol. 177, 4384–4390 (2006).
Christmas, T.J. Lymphocyte sub-populations in the bladder wall in normal bladder, bacterial cystitis and interstitial cystitis. Br. J. Urol. 73, 508–515 (1994).
Harrington, D.S., Fall, M. & Johansson, S.L. Interstitial cystitis: bladder mucosa lymphocyte immunophenotyping and peripheral blood flow cytometry analysis. J. Urol. 144, 868–871 (1990).
Erickson, D.R., Belchis, D.A. & Dabbs, D.J. Inflammatory cell types and clinical features of interstitial cystitis. J. Urol. 158 (3 Pt 1), 790–793 (1997).
MacDermott, J.P., Miller, C.H., Levy, N. & Stone, A.R. Cellular immunity in interstitial cystitis. J. Urol. 145, 274–278 (1991).
Theofilopoulos, A.N., Dummer, W. & Kono, D.H. T cell homeostasis and systemic autoimmunity. J. Clin. Invest. 108, 335–340 (2001).
Sant, A.J. Endogenous antigen presentation by MHC class II molecules. Immunol. Res. 13, 253–267 (1994).
Elyaman, W. et al. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am. J. Pathol. 173, 411–422 (2008).
Cantor, J. & Haskins, K. Effector function of diabetogenic CD4 Th1 T cell clones: a central role for TNF-alpha. J. Immunol. 175, 7738–7745 (2005).
Skapenko, A., Leipe, J., Lipsky, P.E. & Schulze-Koops, H. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther. 7 (Suppl 2), S4–S14 (2005).
Suk, K. et al. IFN-γ/TNF-α synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic b ell death. J. Immunol. 166, 4481–4489 (2001).
Wu, Z., Podack, E.R., McKenzie, J.M., Olsen, K.J. & Zakarija, M Perforin expression by thyroid-infiltrating T cells in autoimmune thyroid disease. Clin. Exp. Immunol. 98, 470–477 (1994).
Chervonsky, A.V. Apoptotic and effector pathways in autoimmunity. Curr. Opin. Immunol. 11, 684–688 (1999).
Bullock, A.D., Becich, M.J., Klutke, C.G. & Ratliff, T.L. Experimental autoimmune cystitis: a potential murine model for ulcerative interstitial cystitis. J. Urol. 148, 1951–1956 (1992).
Luber-Narod, J. et al. Experimental autoimmune cystitis in the Lewis rat: a potential animal model for interstitial cystitis. Urol. Res. 24, 367–373 (1996).
Mitra, S., Dagher, A., Kage, R., Dagher, R.K. & Luber-Narod, J. Experimental autoimmune cystitis: further characterization and serum autoantibodies. Urol. Res. 27, 351–356 (1999).
Lin, Y.H. et al. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. Br. J. Urol. Int. 102, 1724–1730 (2008).
Phull, H., Salkini, M., Purves, T., Funk, J., Copeland, D. & Comiter, C.V. Angiotensin II plays a role in acute murine experimental autoimmune cystitis. Br. J. Urol. Int. 100, 664–667 (2007).
Liu, W., Evanoff, D.P., Chen, X. & Luo, Y . Urinary bladder epithelium antigen induces CD8+ T cell tolerance, activation, and autoimmune response. J. Immunol. 178, 539–546 (2007).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Robertson, J.M., Jensen, P.E. & Evavold, B.D DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323-339 epitope. J. Immunol. 164, 4706–4712 (2000).
Ishikawa, S. et al. A subset of CD4+ T cells expressing early activation antigen CD69 in murine lupus: possible abnormal regulatory role for cytokine imbalance. J. Immunol. 161, 1267–1273 (1998).
Elzey, B.D., Siemens, D.R., Ratliff, T.L. & Lubaroff, D.M Immunization with type 5 adenovirus recombinant for a tumor antigen in combination with recombinant canarypox virus (ALVAC) cytokine gene delivery induces destruction of established prostate tumors. Int. J. Cancer 94, 842–849 (2001).
Kurts, C. et al. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J. Exp. Med. 186, 2057–2062 (1997).
Na, S.Y. et al. Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain 131 (Pt 9), 2353–2365 (2008).
Cao, Y. et al. Induction of experimental autoimmune encephalomyelitis in transgenic mice expressing ovalbumin in oligodendrocytes. Eur. J. Immunol. 36, 207–215 (2006).
Derkow, K. et al. Differential priming of CD8 and CD4 T-cells in animal models of autoimmune hepatitis and cholangitis. Hepatology 46, 1155–1165 (2007).
Camacho, S.A. et al. A key role for ICAM-1 in generating effector cells mediating inflammatory responses. Nat. Immunol. 2, 523–529 (2001).
Xing, Z., Wang, J., Croitoru, K. & Wakeham, J Protection by CD4 or CD8 T cells against pulmonary mycobacterium bovis Bacillus Calmette-Guérin infection. Infect. Immun. 66, 5537–5542 (1998).
Hornquist, E., Grdi, C.D., Mak, T. & Lycke, N CD8-deficient mice exhibit augmented mucosal immune responses and intact adjuvant effects to cholera toxin. Immunology 87, 220–229 (1996).
Griffiths, H.R. Is the generation of neo-antigenic determinants by free radicals central to the development of autoimmune rheumatoid disease? Autoimmune Rev. 7, 544–549 (2008).
McMahon, E.J., Bailey, S.L., Castenada, C.V., Waldner, H. & Miller, S.D Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 11, 335–339 (2005).
Vanderlugt, C.L. & Miller, S.D. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2, 85–95 (2002).
Pichurin, P.N., Chen, C.R., Nagayama, Y., Pichurina, O., Rapoport, B. & McLachlan, S.M. Evidence that factors other than particular thyrotropin receptor T cell epitopes contribute to the development of hyperthyroidism in murine Graves’ disease. Clin. Exp. Immunol. 135, 391–397 (2004).
Yu, M., Kinkel, R.P., Weinstock-Guttman, B., Cook, D.J. & Tuohy, V.K. HLA-DP: a class II restriction molecule involved in epitope spreading during the development of multiple sclerosis. Hum. Immunol. 59, 15–24 (1998).
Liu, W., Deyoung, B.R., Chen, X., Evanoff, D.P. & Luo, Y. RDP58 inhibits T cell-mediated bladder inflammation in an autoimmune cystitis model. J. Autoimmun. 30, 257–265 (2008).
Winzler, C. et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185, 317–328 (1997).
Acknowledgements
We thank Ms Kris Greiner for editorial review of this manuscript. This work was supported in part by the National Institute of Health grant RO1DK066079 and the Department of Defense Peer Reviewed Medical Research Program Award W81XWH-04-1-0070 to Y.L. and by the American Urological Association Foundation Research Scholar Grant, with the following contributors, Interstitial Cystitis Association and Ortho Women's Health and Urology, to W.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, W., Chen, X., Evanoff, D. et al. Urothelial antigen-specific CD4+ T cells function as direct effector cells and induce bladder autoimmune inflammation independent of CD8+ T cells. Mucosal Immunol 4, 428–437 (2011). https://doi.org/10.1038/mi.2010.90
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2010.90