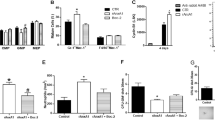
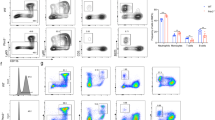
Abstract
Dipeptidylpeptidase 4 (DPP4/CD26) enzymatically cleaves select penultimate amino acids of proteins, including colony-stimulating factors (CSFs), and has been implicated in cellular regulation. To better understand the role of DPP4 regulation of hematopoiesis, we analyzed the activity of DPP4 on the surface of immature blood cells and then comparatively assessed the interactions and functional effects of full-length (FL) and DPP4 truncated (T) factors (T-granulocyte–macrophage-CSF (T-GM-CSF)) and T-interleukin-3 (T-IL-3)) on both in vitro and in vivo models of normal and leukemic cells. T-GM-CSF and -IL-3 had enhanced receptor binding, but decreased CSF activity, compared with their FL forms. Importantly, T-GM-CSF and -IL-3 significantly, and reciprocally, blunted receptor binding and myeloid progenitor cell proliferation activity of both FL-GM-CSF and -IL-3 in vitro and in vivo. Similar effects were apparent in vitro using cluster-forming cells from patients with acute myeloid leukemia regardless of cytogenetic or molecular alterations and in vivo using animal models of leukemia. This suggests that DPP4 T-molecules have modified binding and functions compared with their FL counterparts and may serve regulatory roles in normal and malignant hematopoiesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ou X, O'Leary HA, Broxmeyer HE . Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood 2013; 122: 161–169.
O'Leary H, Ou X, Broxmeyer HE . The role of dipeptidyl peptidase 4 in hematopoiesis and transplantation. Curr Opin Hematol 2013; 20: 314–319.
Broxmeyer HE, Hoggatt J, O'Leary HA, Mantel C, Chitteti BR, Cooper S et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med 2012; 18: 1786–1796.
Favreau AJ, Sathyanarayana P . miR-590-5p, miR-219-5p, miR-15b and miR-628-5p are commonly regulated by IL-3, GM-CSF and G-CSF in acute myeloid leukemia. Leuk Res 2012; 36: 334–341.
Miller PH, Cheung AM, Beer PA, Knapp DJ, Dhillon K, Rabu G et al. Enhanced normal short-term human myelopoiesis in mice engineered to express human-specific myeloid growth factors. Blood 2013; 121: e1–e4.
Riccioni R, Diverio D, Riti V, Buffolino S, Mariani G, Boe A et al. Interleukin (IL)-3/granulocyte macrophage-colony stimulating factor/IL-5 receptor alpha and beta chains are preferentially expressed in acute myeloid leukaemias with mutated FMS-related tyrosine kinase 3 receptor. Br J Haematol 2009; 144: 376–387.
Schiller GJ . High-risk acute myelogenous leukemia: treatment today... and tomorrow. Am Soc Hematol Educ Program 2013; 2013: 201–208.
Thomas D, Powell JA, Green BD, Barry EF, Ma Y, Woodcock J et al. Protein kinase activity of phosphoinositide 3-kinase regulates cytokine-dependent cell survival. PLoS Biol 2013; 11: e1001515.
Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS et al. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev 2012; 250: 277–302.
Onetto-Pothier N, Aumont N, Haman A, Park L, Clark SC, De Lean A et al. IL-3 inhibits the binding of GM-CSF to AML blasts, but the two cytokines act synergistically in supporting blast proliferation. Leukemia 1990; 4: 329–336.
Taketazu F, Chiba S, Shibuya K, Kuwaki T, Tsumura H, Miyazono K et al. IL-3 specifically inhibits GM-CSF binding to the higher affinity receptor. J Cell Physiol 1991; 146: 251–257.
Broxmeyer HE, Williams DE, Cooper S, Shadduck RK, Gillis S, Waheed A et al. Comparative effects in vivo of recombinant murine interleukin 3, natural murine colony-stimulating factor-1, and recombinant murine granulocyte–macrophage colony-stimulating factor on myelopoiesis in mice. J Clin Invest 1987; 79: 721–730.
Broxmeyer HE, Williams DE, Hangoc G, Cooper S, Gillis S, Shadduck RK et al. Synergistic myelopoietic actions in vivo after administration to mice of combinations of purified natural murine colony-stimulating factor 1, recombinant murine interleukin 3, and recombinant murine granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci USA 1987; 84: 3871–3875.
Williams DE, Straneva JE, Cooper S, Shadduck RK, Waheed A, Gillis S et al. Interactions between purified murine colony-stimulating factors (natural CSF-1, recombinant GM-CSF, and recombinant IL-3) on the in vitro proliferation of purified murine granulocyte-macrophage progenitor cells. Exp Hematol 1987; 15: 1007–1012.
Williams DE, Straneva JE, Shen RN, Broxmeyer HE . Purification of murine bone-marrow-derived granulocyte-macrophage colony-forming cells. Exp Hematol 1987; 15: 243–250.
Elbaz O, Shaltout A . Implication of granulocyte–macrophage colony stimulating factor (GM-CSF) and interleukin-3 (IL-3) in children with acute myeloid leukaemia (AML); malignancy. Hematology 2001; 5: 383–388.
Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000; 14: 1777–1784.
Christopherson KW II, Hangoc G, Mantel CR, Broxmeyer HE . Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004; 305: 1000–1003.
Lee BH, Tothova Z, Levine RL, Anderson K, Buza-Vidas N, Cullen DE et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell 2007; 12: 367–380.
Xu D, Liu X, Yu WM, Meyerson HJ, Guo C, Gerson SL et al. Non-lineage/stage-restricted effects of a gain-of-function mutation in tyrosine phosphatase Ptpn11 (Shp2) on malignant transformation of hematopoietic cells. J Exp Med 2011; 208: 1977–1988.
Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 2005; 201: 1307–1318.
Gotoh A, Takahira H, Mantel C, Litz-Jackson S, Boswell HS, Broxmeyer HE . Steel factor induces serine phosphorylation of Stat3 in human growth factor-dependent myeloid cell lines. Blood 1996; 88: 138–145.
Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol 1989; 140: 323–334.
Broxmeyer HE, Grossbard E, Jacobsen N, Moore MA . Evidence for a proliferative advantage of human leukemia colony-forming cells in vitro. J Natl Cancer Inst 1978; 60: 513–521.
Broxmeyer HE, Grossbard E, Jacobsen N, Moore MA . Persistence of inhibitory activity against normal bone-marrow cells during remission of acute leukemia. N Engl J Med 1979; 301: 346–351.
Moore MA, Spitzer G, Williams N, Metcalf D, Buckley J . Agar culture studies in 127 cases of untreated acute leukemia: the prognostic value of reclassification of leukemia according to in vitro growth characteristics. Blood 1974; 44: 1–18.
Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood 2011; 117: 4773–4777.
Christopherson KW, Hangoc G, Broxmeyer HE . Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol 2002; 169: 7000–7008.
Doulatov S, Notta F, Laurenti E, Dick JE . Hematopoiesis: a human perspective. Cell Stem Cell 2012; 10: 120–136.
Niu L, Golde DW, Vera JC, Heaney ML . Kinetic resolution of two mechanisms for high-affinity granulocyte–macrophage colony-stimulating factor binding to its receptor. Blood 1999; 94: 3748–3753.
Woodcock JM, Bagley CJ, Zacharakis B, Lopez AF . A single tyrosine residue in the membrane-proximal domain of the granulocyte–macrophage colony-stimulating factor, interleukin (IL)-3, and IL-5 receptor common beta-chain is necessary and sufficient for high affinity binding and signaling by all three ligands. J Biol Chem 1996; 271: 25999–26006.
Lemoli RM, Gulati SC, Strife A, Lambek C, Perez A, Clarkson BD . Proliferative response of human acute myeloid leukemia cells and normal marrow enriched progenitor cells to human recombinant growth factors IL-3, GM-CSF and G-CSF alone and in combination. Leukemia 1991; 5: 386–391.
Feuring-Buske M, Gerhard B, Cashman J, Humphries RK, Eaves CJ, Hogge DE . Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient NOD/SCID mice and in NOD/SCID mice transgenic for human growth factors. Leukemia 2003; 17: 760–763.
Vellenga E, Ostapovicz D, O'Rourke B, Griffin JD . Effects of recombinant IL-3, GM-CSF, and G-CSF on proliferation of leukemic clonogenic cells in short-term and long-term cultures. Leukemia 1987; 1: 584–589.
Wunderlich M, Chou FS, Link KA, Mizukawa B, Perry RL, Carroll M et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 2010; 24: 1785–1788.
Acknowledgements
These studies were supported by (1) US Public Health Service Grants from the National Institutes of Health: R01HL112669, R01HL67384, R01HL56416, P01DK90948 and U54DK106846 (to HEB) and (2) Indiana University Bioinformatics Core Proposal Grant (to HAO). HAO and MC were supported by NIH T32DK07519 (HEB). We thank Giao Hangoc for help with some of the experiments and Heath D Skinner for clinical insight.
Author contributions
HAO provided concepts, designed and performed experiments and wrote the manuscript. CM, SC and MC performed experiments, HSB provided clinical samples/knowledge, RK, BR, RC, LD and CKQ provided mouse models of leukemia and insight into their use. HEB provided concepts, designed and performed experiments and helped in writing and editing of the manuscript. All authors read the manuscript and provided feedback for clarity and context.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
HEB is a member of the Medical Scientific Advisory Board (MSAB) of Corduse, a cord blood banking company.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
O’Leary, H., Capitano, M., Cooper, S. et al. DPP4 truncated GM-CSF and IL-3 manifest distinct receptor-binding and regulatory functions compared with their full-length forms. Leukemia 31, 2468–2478 (2017). https://doi.org/10.1038/leu.2017.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.98