Abstract
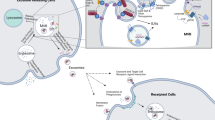
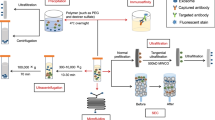
Exosomes are small (30–150 nm) membranous vesicles of endocytic origin produced by all cells under physiological and pathological conditions. They have recently emerged as vehicles for intercellular transfer of molecular and genetic contents from parent to recipient cells. Exosome-mediated transfer of proteins or genes (RNA, miRNA, DNA) results in reprogramming of recipient cell functions. Exosomes carry and deliver information that is essential for health, and they participate in pathological events, including malignant transformation. Within the hematopoietic system, exosomes maintain crosstalk between cells located in the bone marrow compartment and at distant tissue sites. In hematological malignancies, tumor-derived exosomes (TEX) reprogram the bone marrow environment, suppress anti-leukemia immunity, mediate drug resistance and interfere with immunotherapies. TEX are also viewed as promising biomarkers of malignant progression and as potential therapeutic targets. The involvement of TEX in nearly all aspects of malignant transformation has generated much interest in their biology, mechanisms responsible for information transfer and the role they play in cancer escape from the host immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
O'Hayre M, Salanga CL, Handel TM, Allen SJ . Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J 2008; 409: 635–649.
Berleman J, Auer M . The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ Microbiol 2013; 15: 347–354.
Vyas N, Dhawan J . Exosomes: mobile platforms for targeted and synergistic signaling across cell boundaries. Cell Mol Life Sci 2017; 74: 1567–1576.
Boyiadzis M, Whiteside TL . Information transfer by exosomes: a new frontier in hematologic malignancies. Blood Rev 2015; 29: 281–290.
Bogdanov VY, Versteeg HH . 'Soluble tissue factor' in the 21st century: definitions, biochemistry, and pathophysiological role in thrombus formation. Semin Thromb Hemost 2015; 41: 700–707.
Pan BT, Johnstone RM . Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983; 33: 967–978.
Trams EG, Lauter CJ, Salem N Jr, Heine U . Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 1981; 645: 63–70.
Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P . Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med 2011; 9: 86.
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 2016; 113: E968–E977.
Cocucci E, Meldolesi J . Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 2015; 25: 364–372.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO . Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659.
Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 2014; 289: 3869–3875.
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ . Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006; 20: 1487–1495.
Basu J, Ludlow JW . Exosomes for repair, regeneration and rejuvenation. Expert Opin Biol Ther 2016; 16: 489–506.
Kumar B, Garcia M, Murakami JL, Chen CC . Exosome-mediated microenvironment dysregulation in leukemia. Biochim Biophys Acta 2016; 1863: 464–470.
Toth B, Lok CA, Boing A, Diamant M, van der Post JA, Friese K et al. Microparticles and exosomes: impact on normal and complicated pregnancy. Am J Reprod Immunol 2007; 58: 389–402.
Zhang HG, Grizzle WE . Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol 2014; 184: 28–41.
Teow SY, Nordin AC, Ali SA, Khoo AS . Exosomes in human immunodeficiency virus type I pathogenesis: threat or opportunity? Adv Virol 2016; 2016: 9852494.
Tan L, Wu H, Liu Y, Zhao M, Li D, Lu Q . Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity 2016; 49: 357–365.
Vella LJ, Hill AF, Cheng L . Focus on extracellular vesicles: exosomes and their role in protein trafficking and biomarker potential in alzheimer's and parkinson's disease. Int J Mol Sci 2016; 17: 173.
Lee H, Zhang D, Zhu Z, Dela Cruz CS, Jin Y . Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep 2016; 6: 35250.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883–891.
Corcoran C, Rani S, O'Brien K, O'Neill A, Prencipe M, Sheikh R et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One 2012; 7: e50999.
Hong CS, Muller L, Boyiadzis M, Whiteside TL . Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One 2014; 9: e103310.
Choi D, Lee TH, Spinelli C, Chennakrishnaiah S, D'Asti E, Rak J . Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin Cell Dev Biol 2017; e-pub ahead of print 8 January 2017doi:10.1016/j.semcdb.2017.01.003.
Abels ER, Breakefield XO . Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 2016; 36: 301–312.
Mulcahy LA, Pink RC, Carter DR . Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 2014; 3: 24641.
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013; 126: 5553–5565.
Taylor DD, Shah S . Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015; 87: 3–10.
Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C . Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles 2012; 1: 18397.
Muller L, Hong CS, Stolz DB, Watkins SC, Whiteside TL . Isolation of biologically-active exosomes from human plasma. J Immunol Methods 2014; 411: 55–65.
Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL . Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles 2016; 5: 29289.
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015; 523: 177–182.
Mizutani K, Terazawa R, Kameyama K, Kato T, Horie K, Tsuchiya T et al. Isolation of prostate cancer-related exosomes. Anticancer Res 2014; 34: 3419–3423.
Leong SR, Sukumaran S, Hristopoulos M, Totpal K, Stainton S, Lu E et al. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood 2017; 129: 609–618.
Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL . Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res 2005; 11: 1010–1020.
Carrasco-Ramirez P, Greening DW, Andres G, Gopal SK, Martin-Villar E, Renart J et al. Podoplanin is a component of extracellular vesicles that reprograms cell-derived exosomal proteins and modulates lymphatic vessel formation. Oncotarget 2016; 7: 16070–16089.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008; 10: 619–624.
Funk S, Floros T, Hong CS, Jackson EK, Lang S, Whiteside TL . Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res 2016; (in press).
Vlassov AV, Magdaleno S, Setterquist R, Conrad R . Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012; 1820: 940–948.
Nawaz M, Camussi G, Valadi H, Nazarenko I, Ekstrom K, Wang X et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol 2014; 11: 688–701.
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 2016; 428: 688–692.
Gross JC, Chaudhary V, Bartscherer K, Boutros M . Active Wnt proteins are secreted on exosomes. Nat Cell Biol 2012; 14: 1036–1045.
Kalra H, Drummen GP, Mathivanan S . Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci 2016; 17: 170.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10: 1470–1476.
Lazaro-Ibanez E, Sanz-Garcia A, Visakorpi T, Escobedo-Lucea C, Siljander P, Ayuso-Sacido A et al. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate 2014; 74: 1379–1390.
Silva M, Melo SA . Non-coding RNAs in exosomes: new players in cancer biology. Curr Genomics 2015; 16: 295–303.
Raimondo S, Saieva L, Corrado C, Fontana S, Flugy A, Rizzo A et al. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun Signal 2015; 13: 8.
King HW, Michael MZ, Gleadle JM . Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012; 12: 421.
Syn N, Wang L, Sethi G, Thiery JP, Goh BC . Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci 2016; 37: 606–617.
Corrado C, Raimondo S, Saieva L, Flugy AM, De Leo G, Alessandro R . Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an interleukin 8-dependent survival of leukemia cells. Cancer Lett 2014; 348: 71–76.
Huang Z, Feng Y . Exosomes derived from hypoxic colorectal cancer cells promotes angiogenesis through Wnt4 induced beta-catenin signaling in endothelial cells. Oncol Res 2016; e-pub ahead of print 5 October 2016 doi:10.3727/096504016X14752792816791.
Whiteside TL . Exosomes and tumor-mediated immune suppression. J Clin Invest 2016; 126: 1216–1223.
Kahlert C, Kalluri R . Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013; 91: 431–437.
Whiteside TL . Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans 2013; 41: 245–251.
Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL . Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep 2016; 6: 20254.
Robbins PD, Morelli AE . Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14: 195–208.
Hong CS, Muller L, Whiteside TL, Boyiadzis M . Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Front Immunol 2014; 5: 160.
Caivano A, Laurenzana I, De Luca L, La Rocca F, Simeon V, Trino S et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumour Biol 2015; 36: 9739–9752.
Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M . Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 2011; 96: 1302–1309.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183: 1161–1172.
Harshman SW, Canella A, Ciarlariello PD, Rocci A, Agarwal K, Smith EM et al. Characterization of multiple myeloma vesicles by label-free relative quantitation. Proteomics 2013; 13: 3013–3029.
Lima LG, Leal AC, Vargas G, Porto-Carreiro I, Monteiro RQ . Intercellular transfer of tissue factor via the uptake of tumor-derived microvesicles. Thromb Res 2013; 132: 450–456.
Huan J, Hornick NI, Shurtleff MJ, Skinner AM, Goloviznina NA, Roberts CT Jr et al. RNA trafficking by acute myelogenous leukemia exosomes. Cancer Res 2013; 73: 918–929.
Huan J, Hornick NI, Goloviznina NA, Kamimae-Lanning AN, David LL, Wilmarth PA et al. Coordinate regulation of residual bone marrow function by paracrine trafficking of AML exosomes. Leukemia 2015; 29: 2285–2295.
Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH . Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013; 32: 2747–2755.
Tomasetti M, Lee W, Santarelli L, Neuzil J . Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med 2017; 49: e285.
Yang S, Che SP, Kurywchak P, Tavormina JL, Gansmo LB, Correa de Sampaio P et al. Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer. Cancer Biol Ther 2017; 18: 158–165.
Chiarini F, Lonetti A, Evangelisti C, Buontempo F, Orsini E, Evangelisti C et al. Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: from biology to therapeutic targeting. Biochim Biophys Acta 2016; 1863: 449–463.
Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2011; 2: 180.
Johnson SM, Dempsey C, Chadwick A, Harrison S, Liu J, Di Y et al. Metabolic reprogramming of bone marrow stromal cells by leukemic extracellular vesicles in acute lymphoblastic leukemia. Blood 2016; 128: 453–456.
Ohyashiki JH, Umezu T, Ohyashiki K . Exosomes promote bone marrow angiogenesis in hematologic neoplasia: the role of hypoxia. Curr Opin Hematol 2016; 23: 268–273.
Chen Y, Jacamo R, Konopleva M, Garzon R, Croce C, Andreeff M . CXCR4 downregulation of let-7a drives chemoresistance in acute myeloid leukemia. J Clin Invest 2013; 123: 2395–2407.
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest 2013; 123: 1542–1555.
Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A et al. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res 2009; 15: 3325–3332.
Gabrielsson S, Scheynius A . Exosomes in immunity and cancer-friends or foes? Semin Cancer Biol 2014; 28: 1–2.
Yao Y, Wang C, Wei W, Shen C, Deng X, Chen L et al. Dendritic cells pulsed with leukemia cell-derived exosomes more efficiently induce antileukemic immunities. PLoS One 2014; 9: e91463.
Shen C, Hao SG, Zhao CX, Zhu J, Wang C . Antileukaemia immunity: effect of exosomes against NB4 acute promyelocytic leukaemia cells. J Int Med Res 2011; 39: 740–747.
Qazi KR, Gehrmann U, Domange Jordo E, Karlsson MC, Gabrielsson S . Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood 2009; 113: 2673–2683.
Mao L, Li J, Chen WX, Cai YQ, Yu DD, Zhong SL et al. Exosomes decrease sensitivity of breast cancer cells to adriamycin by delivering microRNAs. Tumour Biol 2016; 37: 5247–5256.
Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther 2005; 4: 1595–1604.
Wojtuszkiewicz A, Schuurhuis GJ, Kessler FL, Piersma SR, Knol JC, Pham TV et al. Exosomes secreted by apoptosis-resistant acute myeloid leukemia (AML) blasts harbor regulatory network proteins potentially involved in antagonism of apoptosis. Mol Cell Proteomics 2016; 15: 1281–1298.
Wang J, Hendrix A, Hernot S, Lemaire M, De Bruyne E, Van Valckenborgh E et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014; 124: 555–566.
Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One 2014; 9: e88193.
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 2012; 227: 658–667.
Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci USA 2011; 108: 15336–15341.
Whiteside TL . Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem 2016; 74: 103–141.
Boyiadzis M, Hong CS, Whiteside TL . Circulating exosomes carrying an immunosuppressive cargo interfere with adoptive cell therapy in acute myeloid leukemia. Blood 2016; 128: 1609.
Boyiadzis M, Whiteside TL . Plasma-derived exosomes in acute myeloid leukemia for detection of minimal residual disease: are we ready? Expert Rev Mol Diagn 2016; 16: 623–629.
Whiteside TL . The potential of tumor-derived exosomes for noninvasive cancer monitoring. Expert Rev Mol Diagn 2015; 15: 1293–1310.
Zhu X, You Y, Li Q, Zeng C, Fu F, Guo A et al. BCR-ABL1-positive microvesicles transform normal hematopoietic transplants through genomic instability: implications for donor cell leukemia. Leukemia 2014; 28: 1666–1675.
Zhou J, Wang S, Sun K, Chng WJ . The emerging roles of exosomes in leukemogeneis. Oncotarget 2016; 7: 50698–50707.
Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A . Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 2015; 6: 715–731.
Cai J, Wu G, Jose PA, Zeng C . Functional transferred DNA within extracellular vesicles. Exp Cell Res 2016; 349: 179–183.
Farahani M, Rubbi C, Liu L, Slupsky JR, Kalakonda N . CLL exosomes modulate the transcriptome and behaviour of recipient stromal cells and are selectively enriched in miR-202-3p. PLoS One 2015; 10: e0141429.
Yeh YY, Ozer HG, Lehman AM, Maddocks K, Yu L, Johnson AJ et al. Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood 2015; 125: 3297–3305.
Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 2015; 126: 1106–1117.
Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE . Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood 2010; 115: 1755–1764.
Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH . Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem 2013; 288: 34343–34351.
Broxmeyer HE, Capitano M, Campbell TB, Hangoc G, Cooper S . Modulation of hematopoietic chemokine effects in vitro and in vivo by DPP-4/CD26. Stem Cells Dev 2016; 25: 575–585.
Vader P, Breakefield XO, Wood MJ . Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med 2014; 20: 385–393.
Acknowledgements
This work was supported in part by National Cancer Institute Grants R01 CA168628 to TLW and R21 CA205644 to TLW and MB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Boyiadzis, M., Whiteside, T. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia 31, 1259–1268 (2017). https://doi.org/10.1038/leu.2017.91
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.91
This article is cited by
-
Diagnostic potential of exosomal extracellular vesicles in oncology
BMC Cancer (2024)
-
Leukemogenesis occurs in a microenvironment enriched by extracellular microvesicles/exosomes: recent discoveries and questions to be answered
Leukemia (2024)
-
A novel therapeutic strategy: the significance of exosomal miRNAs in acute myeloid leukemia
Medical Oncology (2024)
-
Extracellular microvesicles: biologic properties, biogenesis, and applications in leukemia
Molecular and Cellular Biochemistry (2024)
-
Mitochondrial transfer in hematological malignancies
Biomarker Research (2023)