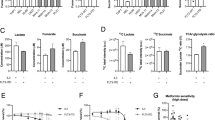
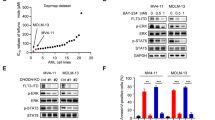
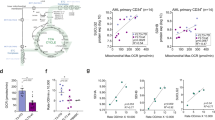
Abstract
Internal tandem duplication (ITD) mutation in Fms-like tyrosine kinase 3 gene (FLT3/ITD) represents an unfavorable genetic change in acute myeloid leukemia (AML) and is associated with poor prognosis. Metabolic alterations have been involved in tumor progression and attracted interest as a target for therapeutic intervention. However, few studies analyzed the adaptations of cellular metabolism in the context of FLT3/ITD mutation. Here, we report that FLT3/ITD causes a significant increase in aerobic glycolysis through AKT-mediated upregulation of mitochondrial hexokinase (HK2), and renders the leukemia cells highly dependent on glycolysis and sensitive to pharmacological inhibition of glycolytic activity. Inhibition of glycolysis preferentially causes severe ATP depletion and massive cell death in FLT3/ITD leukemia cells. Glycolytic inhibitors significantly enhances the cytotoxicity induced by FLT3 tyrosine kinase inhibitor sorafenib. Importantly, such combination provides substantial therapeutic benefit in a murine model bearing FLT3/ITD leukemia. Our study suggests that FLT3/ITD mutation promotes Warburg effect, and such metabolic alteration can be exploited to develop effective therapeutic strategy for treatment of AML with FLT3/ITD mutation via metabolic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kindler T, Lipka DB, Fischer T . FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 2010; 116: 5089–5102.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res 2001; 61: 7233–7239.
Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 2012; 485: 260–263.
Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 2000; 96: 3907–3914.
Rocnik JL, Okabe R, Yu JC, Lee BH, Giese N, Schenkein DP et al. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood 2006; 108: 1339–1345.
Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res 2005; 65: 9643–9650.
Konig H, Levis M . Targeting FLT3 to treat leukemia. Expert Opin Ther Targets 2015; 19: 37–54.
Daver N, Cortes J, Ravandi F, Patel KP, Burger JA, Konopleva M et al. Secondary mutations as mediators of resistance to targeted therapy in leukemia. Blood 2015; 125: 3236–3245.
Chiaradonna F, Sacco E, Manzoni R, Giorgio M, Vanoni M, Alberghina L . Ras-dependent carbon metabolism and transformation in mouse fibroblasts. Oncogene 2006; 25: 5391–5404.
Hu Y, Lu W, Chen G, Wang P, Chen Z, Zhou Y et al. K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res 2012; 22: 399–412.
Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 2011; 7: 523.
Chen Z, Lu W, Garcia-Prieto C, Huang P . The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr 2007; 39: 267–274.
Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ 2014; 21: 124–135.
Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood 2002; 99: 3885–3891.
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 2005; 65: 613–621.
Huang A, Ju HQ, Liu K, Zhan G, Liu D, Wen S et al. Metabolic alterations and drug sensitivity of tyrosine kinase inhibitor resistant leukemia cells with a FLT3/ITD mutation. Cancer Lett 2016; 377: 149–157.
Mathupala SP, Ko YH, Pedersen PL . Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 2006; 25: 4777–4786.
Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC . Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep 2015; 42: 841–851.
Rasola A, Sciacovelli M, Pantic B, Bernardi P . Signal transduction to the permeability transition pore. FEBS Lett 2010; 584: 1989–1996.
Chu SH, Small D . Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updat 2009; 12: 8–16.
Chen Z, Zhang H, Lu W, Huang P . Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta 2009; 1787: 553–560.
Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia 2007; 21: 439–445.
Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst 2008; 100: 184–198.
Ferrara F . New agents for acute myeloid leukemia: is it time for targeted therapies? Expert Opin Investig Drugs 2012; 21: 179–189.
Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD . FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat 2009; 12: 81–89.
El Sayed SM, Mohamed WG, Seddik MA, Ahmed AS, Mahmoud AG, Amer WH et al. Safety and outcome of treatment of metastatic melanoma using 3-bromopyruvate: a concise literature review and case study. Chin J Cancer 2014; 33: 356–364.
Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL . A translational study “case report’ on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr 2012; 44: 163–170.
Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys 1996; 35: 103–111.
Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K et al. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 2010; 70: 1388–1394.
Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 2013; 71: 523–530.
Tse KF, Mukherjee G, Small D . Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia 2000; 14: 1766–1776.
Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell 2002; 9: 1031–1044.
Hu Y, Lu W, Chen G, Wang P, Chen Z, Zhou Y et al. K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res Feb 22: 399–412.
Serkova N, Boros LG . Detection of resistance to imatinib by metabolic profiling: clinical and drug development implications. Am J Pharmacogenomics 2005; 5: 293–302.
Kindler T, Lipka DB, Fischer T . FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 2012; 116: 5089–5102.
Arora KK, Pedersen PL . Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem 1988; 263: 17422–17428.
Crompton M . The mitochondrial permeability transition pore and its role in cell death. Biochem J 1999; 341: 233–249.
Vyssokikh MY, Zorova L, Zorov D, Heimlich G, Jurgensmeier JJ, Brdiczka D . Bax releases cytochrome c preferentially from a complex between porin and adenine nucleotide translocator. Hexokinase activity suppresses this effect. Mol Biol Rep 2002; 29: 93–96.
Vyssokikh MY, Brdiczka D . The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim Pol 2003; 50: 389–404.
Lind JS, Dingemans AM, Groen HJ, Thunnissen FB, Bekers O, Heideman DA et al. A multicenter phase II study of erlotinib and sorafenib in chemotherapy-naive patients with advanced non-small cell lung cancer. Clin Cancer Res 2010; 16: 3078–3087.
Acknowledgements
We thank Dr Donald Small for providing the BaF3/ITD cells. The current study was supported by Guangdong Natural Science Foundation (No. 2014A030313037), National Basic Research Program of China (973 program, No. 2013CB910304), Guangzhou Innovation Research Program (LCY201317) and Guangzhou Technology Program (No. 201508020250).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Ju, HQ., Zhan, G., Huang, A. et al. ITD mutation in FLT3 tyrosine kinase promotes Warburg effect and renders therapeutic sensitivity to glycolytic inhibition. Leukemia 31, 2143–2150 (2017). https://doi.org/10.1038/leu.2017.45
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.45
This article is cited by
-
Crosstalk between autophagy and metabolism: implications for cell survival in acute myeloid leukemia
Cell Death Discovery (2024)
-
The role of bone marrow microenvironment (BMM) cells in acute myeloid leukemia (AML) progression: immune checkpoints, metabolic checkpoints, and signaling pathways
Cell Communication and Signaling (2023)
-
NADPH oxidase mediated oxidative stress signaling in FLT3-ITD acute myeloid leukemia
Cell Death Discovery (2023)
-
Pacritinib inhibits glucose consumption in squamous cell lung cancer cells by targeting FLT3
Scientific Reports (2023)
-
Targeting nucleolin improves sensitivity to chemotherapy in acute lymphoblastic leukemia
Cellular Oncology (2023)