Abstract
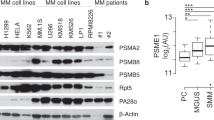
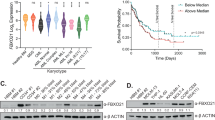
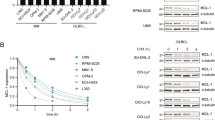
14-3-3 proteins are a family of master regulators of intracellular signaling, yet their impact on proteasome function is unknown. We demonstrate that 14-3-3ζ binds the 11S proteasome activator, limiting proteasome assembly and cellular capacity for protein degradation. To define the functional impact of 14-3-3ζ proteasomal binding in myeloma cells, silencing and overexpression experiments are performed. We find that downregulation of 14-3-3ζ impairs myeloma cell growth and confers resistance to clinically used proteasome inhibitors. In a large cohort of newly diagnosed myeloma patients, elevated expression of 14-3-3ζ is associated with high risk myeloma genetic subtypes and worse prognosis overall. Our work demonstrates the important role of 14-3-3ζ in regulating proteasome function, myeloma cell growth and sensitivity to therapeutics, and suggests regulation of 14-3-3ζ as a new approach in myeloma therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Drexler HC . Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci USA 1997; 94: 855–860.
Voorhees PM, Dees EC, O'Neil B, Orlowski RZ . The proteasome as a target for cancer therapy. Clin Cancer Res 2003; 9: 6316–6325.
Schmidt M, Finley D . Regulation of proteasome activity in health and disease. Biochim Biophys Acta 2014; 1843: 13–25.
Livneh I, Cohen-Kaplan V, Cohen-Rosenzweig C, Avni N, Ciechanover A . The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Res 2016; 26: 869–885.
Fu H, Subramanian RR, Masters SC . 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol 2000; 40: 617–647.
Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H . Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 1997; 277: 1501–1505.
Zha J, Harada H, Yang E, Jockel J, Korsmeyer S-J . Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell 1996; 87: 619–628.
Fu H, Xia K, Pallas DC, Cui C, Conroy K, Narsimhan RP et al. Interaction of the protein kinase Raf-1 with 14-3-3 proteins. Science 1994; 266: 126–129.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999; 96: 857–868.
Maxwell SA, Li Z, Jaye D, Ballard S, Ferrell J, Fu H . 14-3-3ζ mediates resistance of diffuse large B cell lymphoma to an anthracycline-based chemotherapeutic regimen. J Biol Chem 2009; 284: 22379–22389.
Matta A, DeSouza L-V, Ralhan R, Siu K-W . Small interfering RNA targeting 14-3-3ζ increases efficacy of chemotherapeutic agents in head and neck cancer cells. Mol Cancer Ther 2010; 9: 2676–2688.
Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, Boise LH . Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006; 107: 4907–4916.
Bianchi G, Oliva L, Cascio P, Pengo N, Fontana F, Cerruti F et al. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood 2009; 113: 3040–3049.
Li Z, Zhao J, Du Y, Park HR, Sun SY, Bernal-Mizrachi L et al. Down-regulation of 14-3-3zeta suppresses anchorage-independent growth of lung cancer cells through anoikis activation. Proc Natl Acad Sci USA 2008; 105: 162–167.
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J et al. 14-3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res 2009; 69: 3425–3432.
Zhang Z, Krutchinsky A, Endicott S, Realini C, Rechsteiner M, Standing KG . Proteasome activator 11S REG or PA28: recombinant REG alpha/REG beta hetero-oligomers are heptamers. Biochemistry 1999; 38: 5651–5658.
Stadtmueller B-M, Hill C-P . Proteasome activators. Mol Cell 2011; 41: 8–19.
Gomez-Bougie P, Wuillème-Toumi S, Ménoret E, Trichet V, Robillard N, Philippe M et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res 2007; 67: 5418–5424.
Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood 2003; 101: 1530–1534.
Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia 2009; 23: 2210–2221.
Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell 2012; 22: 345–358.
Gu Y, Kaufman JL, Bernal L, Torre C, Matulis SM, Harvey RD et al. MLN4924, an NAE inhibitor, suppresses AKT and mTOR signaling via upregulation of REDD1 in human myeloma cells. Blood 2014; 123: 3269–3276.
Rechsteiner M, Realini C, Ustrell V . The proteasome activator 11S REG(PA28) and class I antigen presentation. Biochem J 2000; 345: 1–15.
Zhang XD, Baladandayuthapani V, Lin H, Mulligan G, Li B, Esseltine DL et al. Tight junction protein 1 modulates proteasome capacity and proteasome inhibitor sensitivity in multiple myeloma via EGFR/JAK1/STAT3 signaling. Cancer Cell 2016; 29: 639–652.
Hoeller D, Hecker C-M, Dikic I . Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer 2006; 6: 776–788.
Masters S-C, Fu H . 14-3-3 proteins mediate an essential anti-apoptotic signal. J Biol Chem 2001; 276: 45193–45200.
Cascio P, Call M, Petre B-M, Walz T, Goldberg A-L . Properties of the hybrid form of the 26S proteasome containing both 19S and PA28 complexes. EMBO J 2002; 21: 2636–2645.
Kasinski A, Dong X, Khuri F-R, Boss J, Fu H . Transcriptional regulation of YWHAZ, the gene encoding 14-3-3ζ. PLoS ONE 2014; 9: e93480.
Murata T, Takayama K, Urano T, Fujimura T, Ashikari D, Obinata D et al. 14-3-3ζ, a novel androgen-responsive gene, is upregulated in prostate cancer and promotes prostate cancer cell proliferation and survival. Clin Cancer Res 2012; 18: 5617–5627.
Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D et al. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 2007; 171: 513–524.
Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB et al. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 2007; 447: 859–863.
Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San Miguel JF . Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high risk multiple myeloma. Blood 2013; 121: 884–892.
Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol 2010; 28: 4630–4634.
Acknowledgements
This work is supported by the Richard and Annelly Deets Myeloma Foundation, and the Levine Family Foundation
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SL is a consultant for Millennium, Onyx, Novartis, BMS, Janssen and Celgene, none of which is relevant for the current manuscript. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Gu, Y., Xu, K., Torre, C. et al. 14-3-3ζ binds the proteasome, limits proteolytic function and enhances sensitivity to proteasome inhibitors. Leukemia 32, 744–751 (2018). https://doi.org/10.1038/leu.2017.288
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.288
This article is cited by
-
NEDD4L binds the proteasome and promotes autophagy and bortezomib sensitivity in multiple myeloma
Cell Death & Disease (2022)
-
Downregulation of PA28α induces proteasome remodeling and results in resistance to proteasome inhibitors in multiple myeloma
Blood Cancer Journal (2020)
-
The dynamic and stress-adaptive signaling hub of 14-3-3: emerging mechanisms of regulation and context-dependent protein–protein interactions
Oncogene (2018)