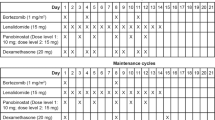
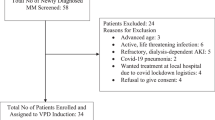
Abstract
Despite therapeutic advances, multiple myeloma remains incurable, with limited options for patients with refractory disease. We conducted a large, multi-cohort clinical trial testing various doses and treatment schedules of pomalidomide and dexamethasone (Pom/dex) in patients with refractory multiple myeloma. Overall, 345 patients were enrolled to six cohorts based on number and type of prior lines of therapy, pomalidomide dose and schedule. Median prior lines of therapy were three with near universal prior exposure to proteasome inhibitors and/or immunomodulatory drugs. A confirmed response rate of 35% was noted for all cohorts (range 23–65%) with higher responses in cohorts with fewer prior lines of therapy. Median time to confirmed response was ⩽2 months and the longest progression-free survival and overall survival seen in any cohort were 13.1 and 47.9 months, respectively. Observed adverse reactions were as expected, with myelosuppression and fatigue being the most common hematologic and non-hematologic adverse events (AEs), respectively. Longer durations of treatment and response, higher response rates and fewer AEs were noted with the 2 mg pomalidomide dose. This is the longest follow-up data for Pom/dex in refractory multiple myeloma and will help shape the real-world utilization of this regimen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015; 372: 142–152.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 1319–1331.
Moreau P, Masszi T, Grazsko N, Bahlis NJ, Hansson M, Pour L et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 374: 1621–1634.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754–766.
Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol 2015; 33: 3459–3466.
Meadows JP, Mark TM . Management of double-refractory multiple myeloma. Curr Hematol Malig Rep 2013; 8: 253–260.
Sonneveld P, Broijl A . Treatment of relapsed and refractory multiple myeloma. Haematologica 2016; 101: 995.
Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 2012; 26: 149–157.
Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA . Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation. Br J Haematol 2008; 141: 41–51.
Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood 2014; 123: 1826–1832.
Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol 2009; 27: 5008–5014.
Lacy MQ, Alfred JB, Gertz MA, Hayman, Short KD, Buadi F et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood 2011; 118: 2970–2975.
Leleu X, Attal M, Arnulf B, Moreau P, Traulie C, Marit G et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009-02. Blood 2013; 121: 1968–1975.
Dimopoulos MA, Palumbo A, Corradini P, Cavo M, Delforge M, Di Ramonodo F et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): a phase 3b study in refractory multiple myeloma. Blood 2016; 128: 497–503.
Siegel DS, Weisel KC, Dimopoulos MS, Baz R, Richardson P, Delforge M et al. Pomalidomide plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and moderate renal impairment: a pooled analysis of three clinical trials. Leuk Lymphoma 2016; 57: 2833–2838.
Lacy MQ, Hayman DR, Gertz MA, Short KD, Dispenzieri A, Kumar S et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia 2010; 24: 1934–1939.
Kyle RA, Rajkumar SV . Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2009; 23: 3–9.
Mikhael JR, Dingli D, Roy V, Reeder CB, Duadi FK, Hayman SR et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 2013; 88: 360–376.
San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 2013; 14: 1055–1066.
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016; 387: 1551–1560.
Song KW, Dimopoulas MA, Weisel KC, Moreau P, Palumbo A, Belch A et al. Health-related quality of life from the MM-003 trial of pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed and/or refractory multiple myeloma. Haematologica 2015; 100: e63–e67.
Weisel K, Dimopoulos M, Song KW, Moreau P, Palumbo A, Belch A et al. Pomalidomide and low-dose dexamethasone improves health-related quality of life and prolongs time to worsening in relapsed/refractory patients with multiple myeloma enrolled in the MM-003 Randomized Phase III Trial. Clin Lymphoma Myeloma Leuk 2015; 15: 519–530.
Acknowledgements
Author contributions
BRL, SK, SVR and MQL designed the clinical trial; SA, JRM, SK, VR, DD, PLB, FKB, SVR, RF, MAG, PK, TS, SRH, AKS, AD, WIG, CBR, YL, RSG, NL, TK, JAL, SJR, AAC-K and MQL conducted the clinical trial; BRL and KML performed the analysis; SA, BRL, KML and MQL wrote the manuscript; and all authors revised and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SA has served on advisory board for Amgen, Novartis, Takeda and Pharmacyclics, and receives research funding from Pharmacyclics; JRM receives research funding from Abbvie, Celgene and Sanofi; SK receives research funding from Takeda, Celgene, Novartis, Abbvie, Janssen and Roche; DD receives research funding from Karyopharm, Amgen and Takeda; PLB receives research funding from Novartis, Constellation and Mundipharma, and has served on advisory board for Incyte, Janssen, Adaptive Bioscience and Juno Therapeutics; MAG receives research funding from Celgene, Janssen, Prothena, Ionis, Alnylam and Sandoz; PK has served on advisory board for Sanofi-Genzyme and receives research funding from Takeda, Celgene and Amgen; AKS receives research funding from Celgene, Amgen, Bristol Myers Squibb and Janssen; AD receives research funding from Celgene, Millennium, Pfizer and Janssen, and has received travel grant from Pfizer and Prothena; CBR receives research funding from Celgene, Novartis, Takeda and BMS; YL receives research funding from Janssen; ACK receives research funding from Pharmacyclics; MQL receives research funding from Celgene. The remaining authors declare no conflict of interest. Funding from CA 186781 was utilized to support investigator effort in data analysis and manuscript preparation for this trial.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Ailawadhi, S., Mikhael, J., LaPlant, B. et al. Pomalidomide–dexamethasone in refractory multiple myeloma: long-term follow-up of a multi-cohort phase II clinical trial. Leukemia 32, 719–728 (2018). https://doi.org/10.1038/leu.2017.258
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.258
This article is cited by
-
Pomalidomide and dexamethasone combination with additional cyclophosphamide in relapsed/refractory multiple myeloma (AMN001)—a trial by the Asian Myeloma Network
Blood Cancer Journal (2019)
-
CD26 is a potential therapeutic target by humanized monoclonal antibody for the treatment of multiple myeloma
Blood Cancer Journal (2018)