Abstract
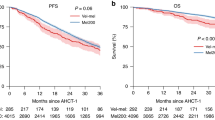
The Dutch-Belgian Cooperative Trial Group for Hematology Oncology Group-65/German-speaking Myeloma Multicenter Group-HD4 (HOVON-65/GMMG-HD4) phase III trial compared bortezomib (BTZ) before and after high-dose melphalan and autologous stem cell transplantation (HDM, PAD arm) compared with classical cytotoxic agents prior and thalidomide after HDM (VAD arm) in multiple myeloma (MM) patients aged 18–65 years. Here, the long-term follow-up and data on second primary malignancies (SPM) are presented. After a median follow-up of 96 months, progression-free survival (censored at allogeneic transplantation, PFS) remained significantly prolonged in the PAD versus VAD arm (hazard ratio (HR)=0.76, 95% confidence interval (95% CI) of 0.65–0.89, P=0.001). Overall survival (OS) was similar in the PAD versus VAD arm (HR=0.89, 95% CI: 0.74–1.08, P=0.24). The incidence of SPM were similar between the two arms (7% each, P=0.73). The negative prognostic effects of the cytogenetic aberration deletion 17p13 (clone size ⩾10%) and renal impairment at baseline (serum creatinine >2 mg dl−1) on PFS and OS remained abrogated in the PAD but not VAD arm. OS from first relapse/progression was similar between the study arms (HR=1.02, P=0.85). In conclusion, the survival benefit with BTZ induction/maintenance compared with classical cytotoxic agents and thalidomide maintenance is maintained without an increased risk of SPM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352: 2487–2498.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015; 372: 142–152.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 2010; 11: 29–37.
Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 2012; 366: 1759–1769.
Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999; 341: 1565–1571.
Miguel JS, Weisel K, Moreau P, Lacy M, Song K, Delforge M et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 2013; 14: 1055–1066.
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373: 1207–1219.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631.
Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res 2001; 61: 3071–3076.
LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res 2002; 62: 4996–5000.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359: 906–917.
Sonneveld P, Goldschmidt H, Rosiñol L, Bladé J, Lahuerta JJ, Cavo M et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol 2013; 31: 3279–3287.
Sonneveld P, Schmidt-Wolf IGH, van der Holt B, El Jarari L, Bertsch U, Salwender H et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol 2012; 30: 2946–2955.
Scheid C, Sonneveld P, Schmidt-Wolf IGH, van der Holt B, Jarari LE, Bertsch U et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica 2014; 99: 148–154.
Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood 2012; 119: 940–948.
Risk of Second Primary Cancers in Multiple Myeloma Survivors in German and Swedish Cancer Registries: Scientific Reports. https://doi.org/file:///Users/MacEli/Library/Application%20Support/Firefox/Profiles/2b3svrdf.default/zotero/storage/9NI58VDW/srep22084.html (accessed 1 Aug2016).
Thomas A, Mailankody S, Korde N, Kristinsson SY, Turesson I, Landgren O . Second malignancies after multiple myeloma: from 1960s to 2010s. Blood 2012; 119: 2731–2737.
Palumbo A, Bringhen S, Kumar SK, Lupparelli G, Usmani S, Waage A et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol 2014; 15: 333–342.
Durie BG, Salmon SE . A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975; 36: 842–854.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol 2013; 31: 448–455.
Greipp PR, San Miguel J, Durie BGM, Crowley JJ, Barlogie B, Bladé J et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420.
Goldschmidt H, Sonneveld P, Cremer FW, van der Holt B, Westveer P, Breitkreutz I et al. Joint HOVON-50/GMMG-HD3 randomized trial on the effect of thalidomide as part of a high-dose therapy regimen and as maintenance treatment for newly diagnosed myeloma patients. Ann Hematol 2003; 82: 654–659.
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F et al. Comparison of 200mg/m2 melphalan and 8Gy total body irradiation plus 140mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood 2002; 99: 731–735.
Bladé J, Samson D, Reece D, Apperley J, BJÖrkstrand B, Gahrton Gö et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol 1998; 102: 1115–1123.
Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473.
Engelhardt M, Terpos E, Kleber M, Gay F, Wäsch R, Morgan G et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica 2014; 99: 232–242.
Moreau P, Attal M, Facon T . Frontline therapy of multiple myeloma. Blood 2015; 125: 3076–3084.
Landgren O, Mailankody S . Update on second primary malignancies in multiple myeloma: a focused review. Leukemia 2014; 28: 1423–1426.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951.
Cavo M, Salwender H, Rosiñol L, Moreau P, Petrucci MT, Blau IW et al. Double vs single autologous stem cell transplantation after bortezomib-based induction regimens for multiple myeloma: an integrated analysis of patient-level data from phase European III studies. Blood 2013; 122: 767–767.
Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 Clinical Study. J Clin Oncol 2007; 25: 2434–2441.
Fermand JP, Marolleau J, Alberti C, Divine M, Leblond V, Macro M et al. In single versus tandem high dose therapy (HDT) supported with autologous blood stem cell (ABSC) transplantation using unselected or CD34 enriched ABSC: preliminary results of a two by two designed randomized trial in 230 young patients with multiple myeloma (MM). Blood 2001; 98: 815a, Abstract #3387.
Mai EK, Benner A, Bertsch U, Brossart P, Hänel A, Kunzmann V et al. Single versus tandem high-dose melphalan followed by autologous blood stem cell transplantation in multiple myeloma: long-term results from the phase III GMMG-HD2 trial. Br J Haematol 2016; 173: 731–741.
Sonneveld P, van der Holt B, Segeren CM, Vellenga E, Croockewit AJ, Verhoef GEG et al. Intermediate-dose melphalan compared with myeloablative treatment in multiple myeloma: long-term follow-up of the Dutch Cooperative Group HOVON 24 trial. Haematologica 2007; 92: 928–935.
Attal M, Harousseau J-L, Facon T, Guilhot F, Doyen C, Fuzibet J-G et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med 2003; 349: 2495–2502.
Mai EK, Bertsch U, Dürig J, Kunz C, Haenel M, Blau IW et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia 2015; 29: 1721–1729.
Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood 2016; 127: 2569–2574.
Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet 2010; 376: 2075–2085.
Rosiñol L, Oriol A, Teruel AI, Hernández D, López-Jiménez J, de la Rubia J et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood 2012; 120: 1589–1596.
Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the intergroupe Francophone du Myélome. J Clin Oncol 2014;: 32: 2712–2717.
Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010; 116: 679–686.
Merz M, Salwender H, Haenel M, Mai EK, Bertsch U, Kunz C et al. Subcutaneous versus intravenous bortezomib in two different induction therapies for newly diagnosed multiple myeloma: an interim analysis from the prospective GMMG-MM5 trial. Haematologica 2015; 100: 964–969.
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 2011; 12: 431–440.
Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol 2010; 28: 4630–4634.
Merz M, Hielscher T, Seckinger A, Hose D, Mai EK, Raab MS et al. Baseline characteristics, chromosomal alterations, and treatment affecting prognosis of deletion 17p in newly diagnosed myeloma. Am J Hematol 2016; 91: E473–E477.
Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia 2014; 28: 690–693.
Ludwig H, Adam Z, Hajek R, Greil R, Tóthová E, Keil F et al. Light chain–induced acute renal failure can be reversed by Bortezomib-Doxorubicin-Dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol 2010; 28: 4635–4641.
Acknowledgements
The investigators thank all participating patients and their families. Further the authors thank the ‘Zentrum zur Koordination klinischer Studien (KKS)’ Heidelberg, Germany and all participating study sites. Supported by the Dutch Cancer Foundation, the German Federal Ministry of Education and Research, and unrestricted Grant No. MMY3003 from Janssen-Cilag-Ortho Biotech. The German-speaking Myeloma Multicenter Group was supported by grants from Novartis, Amgen (No. P2004-0060), Chugai, and Roche.
Author contributions
Conception and design: PS, PB, SZ, EV, GMJB, DH, MvM-K, PWW, HML and HG. Financial support: AP. Administrative support: BvdH, LeJ, UB, HML and HG. Provision of study materials or patients: PS, IGHS-W, HS, SZ, EV, IWB, KCW, SC, GMJB, MS-K, CS, MP, DH, JH, MSR, RR, RMS, M-JK, MvM-K, UD, WL, PWW, HML and HG. Collection and assembly of data: PS, PB, LeJ, UB, HS, SZ, EV, AB, IWB, KCW, JH, MSR, EKM, SC, GMJB, MS-K, CS, MP, AJ, TH, RR, RMS, M-JK, MvM-K, UD, HWL, PY, HML and HG. Data analysis and interpretation: PS, BvdH, LeJ, SZ, MS-K, CS, PY, HML, TH, EKM and HG. Writing of the first manuscript draft: EKM and BvdH. Manuscript editing and writing: all authors. Final approval of manuscript: all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
EKM: Honoraria: Janssen-Cilag, Takeda; Advisory boards: Takeda; Other support (travel grants): Janssen-Cilag, Takeda, Celgene, Onyx and Mundipharma; PS: Research support from Janssen, Celgene, Amgen, Takeda, Karyopharm Honoraria and advisory boards: Janssen, Celgene, Amgen, Takeda, Karyopharm; MSR: Research Support from Novartis, Amgen, Morphosys; Consulting for Novartis, Amgen, Celgene, Janssen; JH: Advisory boards: Celgene, Janssen, Novartis; Speakers honoraria: Celgene, Janssen, BMS, Amgen; Consultancy: Amgen; Travel support: Amgen, BMS, Takeda; Research Support: Novartis, Sanofi; HJS: Honoraria: Janssen, Celgene; Travel support: Janssen, Celgene; KCW: Consultancy: Amgen, Bristol Myers Squibb, Celgene, Novartis, Janssen, Takeda; Honoraria: Amgen, Bristol Myers Squibb, Celgene, Novartis, Janssen, Takeda; Research funding: Celgene, Janssen; IWB: Research grant: Celgene and Janssen; Advisory boards: Janssen, Celgene, Amgen, Takeda, Novartis, BMS; PS: SkylineDx: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; SZ: Celgene: Honoraria, Research Funding; Takeda Millennium: Honoraria, Research Funding; Onyx: Honoraria; Annemiek Broijl: Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Christ of Scheid: Janssen: Honoraria; Celgene: Honoraria; AP: Janssen: Employment; Dirk Hose: Takeda: Other: Travel grant; EngMab AG: Research Funding; M-JK: Takeda Millennium: Research Funding; Janssen: Honoraria, Research Funding; Roche: Honoraria, Research Funding; UD: Alexion: Honoraria; Janssen: Honoraria; HML: Janssen: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; Amgen: Honoraria; Goldschmidt: Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Onyx: Honoraria, Research Funding; Millennium: Honoraria, Research Funding; BMS: Honoraria, Research Funding. The remaining authors declare no conflict of interest.
Additional information
Parts of this manuscript have been presented at the 2015 ASH Annual Meeting, Orlando, Florida, Abstract #27.
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Goldschmidt, H., Lokhorst, H., Mai, E. et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia 32, 383–390 (2018). https://doi.org/10.1038/leu.2017.211
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.211