Abstract
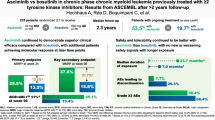
CD33 is variably expressed on leukemia blasts in almost all patients with acute myeloid leukemia (AML) and possibly leukemia stem cells in some. Efforts to target CD33 therapeutically have focused on gemtuzumab ozogamicin (GO; Mylotarg), an antibody-drug conjugate delivering a DNA-damaging calicheamicin derivative. GO is most effective in acute promyelocytic leukemia but induces remissions in other AML types and received accelerated approval in the US in 2000. However, because a large follow-up study showed no survival improvement and increased early deaths the drug manufacturer voluntarily withdrew the US New Drug Application in 2010. More recently, a meta-analysis of data from several trials reported better survival in adults with favorable- and intermediate-risk cytogenetics but not adverse-risk AML randomized to receive GO along with intensive induction chemotherapy. As a result, GO is being re-evaluated by regulatory agencies. Responses to GO are diverse and predictive biological response markers are needed. Besides cytogenetic risk, ATP-binding cassette transporter activity and possibly CD33 display on AML blasts may predict response, but established clinical assays and prospective validation are lacking. Single-nucleotide polymorphisms in CD33 may also be predictive, most notably rs12459419 where the minor T-allele leads to decreased display of full-length CD33 and preferential translation of a splice variant not recognized by GO. Data from retrospective analyses suggest only patients with the rs12459419 CC genotype may benefit from GO therapy but confirmation is needed. Most important may be markers for AML cell sensitivity to calicheamicin, which varies over 100 000-fold, but useful assays are unavailable. Novel CD33-targeted drugs may overcome some of GO’s limitations but it is currently unknown whether such drugs will be more effective in patients benefitting from GO and/or improve outcomes in patients not benefitting from GO, and what the supportive care requirements will be to enable their safe use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cowan AJ, Laszlo GS, Estey EH, Walter RB . Antibody-based therapy of acute myeloid leukemia with gemtuzumab ozogamicin. Front Biosci (Landmark Ed) 2013; 18: 1311–1334.
Macauley MS, Crocker PR, Paulson JC . Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol 2014; 14: 653–666.
Hernández-Caselles T, Martínez-Esparza M, Pérez-Oliva AB, Quintanilla-Cecconi AM, García-Alonso A, Alvarez-López DM et al. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: two isoforms of CD33 are generated by alternative splicing. J Leukoc Biol 2006; 79: 46–58.
Pérez-Oliva AB, Martínez-Esparza M, Vicente-Fernández JJ, Corral-San Miguel R, García-Peñarrubia P, Hernández-Caselles T . Epitope mapping, expression and post-translational modifications of two isoforms of CD33 (CD33M and CD33m) on lymphoid and myeloid human cells. Glycobiology 2011; 21: 757–770.
Malik M, Simpson JF, Parikh I, Wilfred BR, Fardo DW, Nelson PT et al. CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing. J Neurosci 2013; 33: 13320–13325.
Raj T, Ryan KJ, Replogle JM, Chibnik LB, Rosenkrantz L, Tang A et al. CD33: increased inclusion of exon 2 implicates the Ig V-set domain in Alzheimer's disease susceptibility. Hum Mol Genet 2014; 23: 2729–2736.
Schwarz F, Springer SA, Altheide TK, Varki NM, Gagneux P, Varki A . Human-specific derived alleles of CD33 and other genes protect against postreproductive cognitive decline. Proc Natl Acad Sci USA 2016; 113: 74–79.
Laszlo GS, Harrington KH, Gudgeon CJ, Beddoe ME, Fitzgibbon MP, Ries RE et al. Expression and functional characterization of CD33 transcript variants in human acute myeloid leukemia. Oncotarget 2016; 7: 43281–43294.
Lamba JK, Chauhan L, Shin M, Loken M, Pollard J, Wang Y-C et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo AML: report from randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol 2017 doi:10.1200/JCO.2016.71.2513.
Jiang T, Yu JT, Hu N, Tan MS, Zhu XC, Tan L . CD33 in Alzheimer's disease. Mol Neurobiol 2014; 49: 529–535.
Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A et al. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat Neurosci 2013; 16: 848–850.
Malik M, Chiles J 3rd, Xi HS, Medway C, Simpson J, Potluri S et al. Genetics of CD33 in Alzheimer's disease and acute myeloid leukemia. Hum Mol Genet 2015; 24: 3557–3570.
Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 2013; 78: 631–643.
Laszlo GS, Estey EH, Walter RB . The past and future of CD33 as therapeutic target in acute myeloid leukemia. Blood Rev 2014; 28: 143–153.
Khan N, Hills RK, Virgo P, Couzens S, Clark N, Gilkes A et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia 2017; 31: 1059–1068.
Pollard JA, Alonzo TA, Loken M, Gerbing RB, Ho PA, Bernstein ID et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood 2012; 119: 3705–3711.
Krupka C, Kufer P, Kischel R, Zugmaier G, Bögeholz J, Köhnke T et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood 2014; 123: 356–365.
Tanimoto M, Scheinberg DA, Cordon-Cardo C, Huie D, Clarkson BD, Old LJ . Restricted expression of an early myeloid and monocytic cell surface antigen defined by monoclonal antibody M195. Leukemia 1989; 3: 339–348.
Scheinberg DA, Tanimoto M, McKenzie S, Strife A, Old LJ, Clarkson BD . Monoclonal antibody M195: a diagnostic marker for acute myelogenous leukemia. Leukemia 1989; 3: 440–445.
Jilani I, Estey E, Huh Y, Joe Y, Manshouri T, Yared M et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Pathol 2002; 118: 560–566.
Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth MT, Fritsch G et al. Expression of the target receptor CD33 in CD34+/CD38-/CD123+ AML stem cells. Eur J Clin Invest 2007; 37: 73–82.
Mortland L, Alonzo TA, Walter RB, Gerbing RB, Mitra AK, Pollard JA et al. Clinical significance of CD33 nonsynonymous single-nucleotide polymorphisms in pediatric patients with acute myeloid leukemia treated with gemtuzumab-ozogamicin-containing chemotherapy. Clin Cancer Res 2013; 19: 1620–1627.
Guglielmi C, Martelli MP, Diverio D, Fenu S, Vegna ML, Cantu-Rajnoldi A et al. Immunophenotype of adult and childhood acute promyelocytic leukaemia: correlation with morphology, type of PML gene breakpoint and clinical outcome. A cooperative Italian study on 196 cases. Br J Haematol 1998; 102: 1035–1041.
Paietta E . Expression of cell-surface antigens in acute promyelocytic leukaemia. Best Pract Res Clin Haematol 2003; 16: 369–385.
De Propris MS, Raponi S, Diverio D, Milani ML, Meloni G, Falini B et al. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica 2011; 96: 1548–1551.
Ehninger A, Kramer M, Röllig C, Thiede C, Bornhäuser M, von Bonin M et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J 2014; 4: e218.
Pollard JA, Loken M, Gerbing RB, Raimondi SC, Hirsch BA, Aplenc R et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol 2016; 34: 747–755.
Olombel G, Guerin E, Guy J, Perrot JY, Dumezy F, de Labarthe A et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Blood 2016; 127: 2157–2160.
Walter RB, Appelbaum FR, Estey EH, Bernstein ID . Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood 2012; 119: 6198–6208.
Fialkow PJ, Singer JW, Adamson JW, Vaidya K, Dow LW, Ochs J et al. Acute nonlymphocytic leukemia: heterogeneity of stem cell origin. Blood 1981; 57: 1068–1073.
Fialkow PJ, Singer JW, Raskind WH, Adamson JW, Jacobson RJ, Bernstein ID et al. Clonal development, stem-cell differentiation, and clinical remissions in acute nonlymphocytic leukemia. N Engl J Med 1987; 317: 468–473.
Bernstein ID, Singer JW, Andrews RG, Keating A, Powell JS, Bjornson BH et al. Treatment of acute myeloid leukemia cells in vitro with a monoclonal antibody recognizing a myeloid differentiation antigen allows normal progenitor cells to be expressed. J Clin Invest 1987; 79: 1153–1159.
Bernstein ID, Singer JW, Smith FO, Andrews RG, Flowers DA, Petersens J et al. Differences in the frequency of normal and clonal precursors of colony-forming cells in chronic myelogenous leukemia and acute myelogenous leukemia. Blood 1992; 79: 1811–1816.
Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood 2005; 106: 4086–4092.
Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O'Connor J et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol 2005; 23: 4110–4116.
Sekeres MA, Lancet JE, Wood BL, Grove LE, Sandalic L, Sievers EL et al. Randomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia. Haematologica 2013; 98: 119–128.
Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem 2002; 13: 47–58.
Labrijn AF, Buijsse AO, van den Bremer ETJ, Verwilligen AYW, Bleeker WK, Thorpe SJ et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol 2009; 27: 767–771.
Hamann PR, Hinman LM, Beyer CF, Lindh D, Upeslacis J, Flowers DA et al. An anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Choice of linker. Bioconjug Chem 2002; 13: 40–46.
Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 2001; 7: 1490–1496.
Jedema I, Barge RM, van der Velden VH, Nijmeijer BA, van Dongen JJ, Willemze R et al. Internalization and cell cycle-dependent killing of leukemic cells by gemtuzumab ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia 2004; 18: 316–325.
Boghaert ER, Khandke K, Sridharan L, Armellino D, Dougher M, Dijoseph JF et al. Tumoricidal effect of calicheamicin immuno-conjugates using a passive targeting strategy. Int J Oncol 2006; 28: 675–684.
Sissi C, Moro S, Crothers DM . Novel insights on the DNA interaction of calicheamicin gamma(1)(I). Biopolymers 2015; 103: 449–459.
Yamauchi T, Matsuda Y, Tasaki T, Negoro E, Ikegaya S, Takagi K et al. Induction of DNA strand breaks is critical to predict the cytotoxicity of gemtuzumab ozogamicin against leukemic cells. Cancer Sci 2012; 103: 1722–1729.
Sullivan N, Lyne L . Sensitivity of fibroblasts derived from ataxia-telangiectasia patients to calicheamicin gamma 1I. Mutat Res 1990; 245: 171–175.
van Duijn-Goedhart A, Zdzienicka MZ, Sankaranarayanan K, van Buul PP . Differential responses of Chinese hamster mutagen sensitive cell lines to low and high concentrations of calicheamicin and neocarzinostatin. Mutat Res 2000; 471: 95–105.
Elmroth K, Nygren J, Mårtensson S, Ismail IH, Hammarsten O . Cleavage of cellular DNA by calicheamicin gamma1. DNA Repair 2003; 2: 363–374.
Audebert M, Salles B, Calsou P . Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem 2004; 279: 55117–55126.
Bouquet F, Ousset M, Biard D, Fallone F, Dauvillier S, Frit P et al. A DNA-dependent stress response involving DNA-PK occurs in hypoxic cells and contributes to cellular adaptation to hypoxia. J Cell Sci 2011; 124 (Pt 11): 1943–1951.
Goemans BF, Zwaan CM, Vijverberg SJH, Loonen AH, Creutzig U, Hählen K et al. Large interindividual differences in cellular sensitivity to calicheamicin may influence gemtuzumab ozogamicin response in acute myeloid leukemia. Leukemia 2008; 22: 2284–2285.
Pagano L, Fianchi L, Caira M, Rutella S, Leone G . The role of gemtuzumab ozogamicin in the treatment of acute myeloid leukemia patients. Oncogene 2007; 26: 3679–3690.
Breccia M, Lo-Coco F . Gemtuzumab ozogamicin for the treatment of acute promyelocytic leukemia: mechanisms of action and resistance, safety and efficacy. Expert Opin Biol Ther 2011; 11: 225–234.
Hütter ML, Schlenk RF . Gemtuzumab ozogamicin in non-acute promyelocytic acute myeloid leukemia. Expert Opin Biol Ther 2011; 11: 1369–1380.
Takeshita A . Efficacy and resistance of gemtuzumab ozogamicin for acute myeloid leukemia. Int J Hematol 2013; 97: 703–716.
Thol F, Schlenk RF . Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert Opin Biol Ther 2014; 14: 1185–1195.
O'Hear C, Inaba H, Pounds S, Shi L, Dahl G, Bowman WP et al. Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia. Cancer 2013; 119: 4036–4043.
Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 2007; 21: 66–71.
Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol 2016; 34: 972–979.
Burnett AK, Hills RK, Hunter AE, Milligan D, Kell WJ, Wheatley K et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 2013; 27: 75–81.
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015; 126: 291–299.
Morris KL, Adams JA, Liu JA . Effect of gemtuzumab ozogamicin on acute myeloid leukemia blast cells in vitro, as a single agent and combined with other cytotoxic cells. Br J Haematol 2006; 135: 509–512.
Tanaka M, Kano Y, Akutsu M, Tsunoda S, Izumi T, Yazawa Y et al. The cytotoxic effects of gemtuzumab ozogamicin (mylotarg) in combination with conventional antileukemic agents by isobologram analysis in vitro. Anticancer Res 2009; 29: 4589–4596.
Prebet T, Etienne A, Devillier R, Romeo E, Charbonnier A, D'Incan E et al. Improved outcome of patients with low- and intermediate-risk cytogenetics acute myeloid leukemia (AML) in first relapse with gemtuzumab and cytarabine versus cytarabine: results of a retrospective comparative study. Cancer 2011; 117: 974–981.
Hospital MA, Prebet T, Bertoli S, Thomas X, Tavernier E, Braun T et al. Core-binding factor acute myeloid leukemia in first relapse: a retrospective study from the French AML Intergroup. Blood 2014; 124: 1312–1319.
Wattad M, Weber D, Döhner K, Krauter J, Gaidzik VI, Paschka P et al. Impact of salvage regimens on response and overall survival in acute myeloid leukemia with induction failure. Leukemia 2017; 31: 1306–1313.
Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol 2011; 29: 369–377.
Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 2012; 30: 3924–3931.
Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012; 379: 1508–1516.
Delaunay J, Recher C, Pigneux A, Witz F, Vey N, Blanchet O et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR study [abstract]. Blood 2011; 118: 37–38.
Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013; 121: 4854–4860.
Löwenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009; 361: 1235–1248.
Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 2009; 361: 1249–1259.
Burnett AK, Russell NH, Hills RK, Kell J, Cavenagh J, Kjeldsen L et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 2015; 125: 3878–3885.
Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 2014; 15: 986–996.
Burnett A, Cavenagh J, Russell N, Hills R, Kell J, Jones G et al. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: a comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 Trial. Haematologica 2016; 101: 724–731.
Amadori S, Suciu S, Stasi R, Salih HR, Selleslag D, Muus P et al. Sequential combination of gemtuzumab ozogamicin and standard chemotherapy in older patients with newly diagnosed acute myeloid leukemia: results of a randomized phase III trial by the EORTC and GIMEMA consortium (AML-17). J Clin Oncol 2013; 31: 4424–4430.
Brunnberg U, Mohr M, Noppeney R, Dürk HA, Sauerland MC, Müller-Tidow C et al. Induction therapy of AML with ara-C plus daunorubicin versus ara-C plus gemtuzumab ozogamicin: a randomized phase II trial in elderly patients. Ann Oncol 2012; 23: 990–996.
Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol 2014; 32: 3021–3032.
Tarlock K, Alonzo TA, Gerbing RB, Raimondi SC, Hirsch BA, Sung L et al. Gemtuzumab ozogamicin reduces relapse risk in FLT3/ITD acute myeloid leukemia: a report from the Children's Oncology Group. Clin Cancer Res 2016; 22: 1951–1957.
Löwenberg B, Beck J, Graux C, van Putten W, Schouten HC, Verdonck LF et al. Gemtuzumab ozogamicin as postremission treatment in AML at 60 years of age or more: results of a multicenter phase 3 study. Blood 2010; 115: 2586–2591.
Fernandez HF, Sun Z, Litzow MR, Luger SM, Paietta EM, Racevskis J et al. Autologous transplantation gives encouraging results for young adults with favorable-risk acute myeloid leukemia, but is not improved with gemtuzumab ozogamicin. Blood 2011; 117: 5306–5313.
Hasle H, Abrahamsson J, Forestier E, Ha SY, Heldrup J, Jahnukainen K et al. Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: results from NOPHO-AML 2004. Blood 2012; 120: 978–984.
Jager E, van der Velden VHJ, te Marvelde JG, Walter RB, Agur Z, Vainstein V . Targeted drug delivery by gemtuzumab ozogamicin: mechanism-based mathematical model for treatment strategy improvement and therapy individualization. PLoS One 2011; 6: e24265.
Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol 2015; 16: 1295–1305.
Lancet JE, Moseley A, Komrokji RS, Coutre SE, DeAngelo DJ, Tallman MS et al. ATRA, arsenic trioxide (ATO), and gemtuzumab ozogamicin (GO) is safe and highly effective in patinets with previously untreated high-risk acute promyelocytic leukemia (APL): final results of the SWOG/Alliance/ECOG S0535 trial [abstract]. Blood 2016; 128: 896.
Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 2017; 129: 1275–1283.
Takeshita A, Shinjo K, Naito K, Matsui H, Sahara N, Shigeno K et al. Efficacy of gemtuzumab ozogamicin on ATRA- and arsenic-resistant acute promyelocytic leukemia (APL) cells. Leukemia 2005; 19: 1306–1311.
Pfizer Inc 2010. Available at http://media.pfizer.com/files/products/mylotarg_hcp_letter.pdf (accessed 6 May 2017).
Tanimoto T, Tsubokura M, Mori J, Pietrek M, Ono S, Kami M . Differences in drug approval processes of 3 regulatory agencies: a case study of gemtuzumab ozogamicin. Invest New Drugs 2013; 31: 473–478.
European Medicines Agency. Refusal Assessment Report for Mylotarg, 2008. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000705/WC500070677.pdf (accessed 6 May 2017).
van der Velden VHJ, Boeckx N, Jedema I, te Marvelde JG, Hoogeveen PG, Boogaerts M et al. High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia 2004; 18: 983–988.
Biedermann B, Gil D, Bowen DT, Crocker PR . Analysis of the CD33-related siglec family reveals that Siglec-9 is an endocytic receptor expressed on subsets of acute myeloid leukemia cells and absent from normal hematopoietic progenitors. Leuk Res 2007; 31: 211–220.
Abdool A, Yeh CH, Kantarjian H, O'Brien S, Bruey J, Giles F et al. Circulating CD33 and its clinical value in acute leukemia. Exp Hematol 2010; 38: 462–471.
Linenberger ML, Hong T, Flowers D, Sievers EL, Gooley TA, Bennett JM et al. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood 2001; 98: 988–994.
Pierceall WE, Lena RJ, Medeiros BC, Blake N, Doykan C, Elashoff M et al. Mcl-1 dependence predicts response to vorinostat and gemtuzumab ozogamicin in acute myeloid leukemia. Leuk Res 2014; 38: 564–568.
Rosen DB, Harrington KH, Cordeiro JA, Leung LY, Putta S, Lacayo N et al. AKT signaling as a novel factor associated with in vitro resistance of human AML to gemtuzumab ozogamicin. PLoS One 2013; 8: e53518.
Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID . Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood 2005; 105: 1295–1302.
Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood 2007; 109: 4168–4170.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V . Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12: 253–268.
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7: 12150.
Paubelle E, Marceau A, Zylbersztejn F, Dussiot M, Moura IC, C-L P et al. HFE gene mutation status predicts response to gemtuzumab ozogamicin in AML [abstract]. Blood 2015; 126: 1307.
Middeldorf I, Galm O, Osieka R, Jost E, Herman JG, Wilop S . Sequence of administration and methylation of SOCS3 may govern response to gemtuzumab ozogamicin in combination with conventional chemotherapy in patients with refractory or relapsed acute myelogenous leukemia (AML). Am J Hematol 2010; 85: 477–481.
Leith CP, Kopecky KJ, Chen IM, Eijdems L, Slovak ML, McConnell TS et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood 1999; 94: 1086–1099.
Legrand O, Simonin G, Beauchamp-Nicoud A, Zittoun R, Marie JP . Simultaneous activity of MRP1 and Pgp is correlated with in vitro resistance to daunorubicin and with in vivo resistance in adult acute myeloid leukemia. Blood 1999; 94: 1046–1056.
Legrand O, Zompi S, Perrot JY, Faussat AM, Benderra Z, Chaoui D et al. P-glycoprotein and multidrug resistance associated protein-1 activity in 132 acute myeloid leukemias according to FAB subtypes and cytogenetics risk groups. Haematologica 2004; 89: 34–41.
Seedhouse CH, Grundy M, White P, Li Y, Fisher J, Yakunina D et al. Sequential influences of leukemia-specific and genetic factors on p-glycoprotein expression in blasts from 817 patients entered into the National Cancer Research Network acute myeloid leukemia 14 and 15 trials. Clin Cancer Res 2007; 13: 7059–7066.
Larson RA, Sievers EL, Stadtmauer EA, Löwenberg B, Estey EH, Dombret H et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005; 104: 1442–1452.
Maniecki MB, Hasle H, Friis-Hansen L, Lausen B, Nielsen OJ, Bendix K et al. Impaired CD163-mediated hemoglobin-scavenging and severe toxic symptoms in patients treated with gemtuzumab ozogamicin. Blood 2008; 112: 1510–1514.
McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res 2007; 31: 599–604.
Tallman MS, McDonald GB, DeLeve LD, Baer MR, Cook MN, Graepel GJ et al. Incidence of sinusoidal obstruction syndrome following Mylotarg (gemtuzumab ozogamicin): a prospective observational study of 482 patients in routine clinical practice. Int J Hematol 2013; 97: 456–464.
Magwood-Golston JS, Kessler S, Bennett CL . Evaluation of gemtuzumab ozogamycin associated sinusoidal obstructive syndrome: findings from an academic pharmacovigilance program review and a pharmaceutical sponsored registry. Leuk Res 2016; 44: 61–64.
Godwin CD, McDonald GB, Walter RB . Sinusoidal obstruction syndrome following CD33-targeted therapy in acute myeloid leukemia. Blood 2017; 129: 2330–2332.
Battipaglia G, Labopin M, Candoni A, Fanin R, El Cheikh J, Blaise D et al. Risk of sinusoidal obstruction syndrome in allogeneic stem cell transplantation after prior gemtuzumab ozogamicin treatment: a retrospective study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant 2017; 52: 592–599.
Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood 2003; 102: 1578–1582.
Boghaert ER, Khandke KM, Sridharan L, Dougher M, Dijoseph JF, Kunz A et al. Determination of pharmacokinetic values of calicheamicin-antibody conjugates in mice by plasmon resonance analysis of small (5 μL) blood samples. Cancer Chemother Pharmacol 2008; 61: 1027–1035.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 2016; 375: 740–753.
Guffroy M, Falahatpisheh H, Biddle K, Kreeger J, Obert L, Walters K et al. Liver microvascular injury and thrombocytopenia of antibody-calicheamicin conjugates in cynomolgus monkeys - mechanism and monitoring. Clin Cancer Res 2017; 23: 1760–1770.
Lapusan S, Vidriales MB, Thomas X, de Botton S, Vekhoff A, Tang R et al. Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs 2012; 30: 1121–1131.
Borthakur G, Rosenblum MG, Talpaz M, Daver N, Ravandi F, Faderl S et al. Phase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignancies. Haematologica 2013; 98: 217–221.
Kung Sutherland MS, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H et al. SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013; 122: 1455–1463.
Watkins KM, Walker R, Fishkin N, Audette C, Kovtun Y, Romanelli A . IMGN779, a CD33-targeted antibody-drug conjugate (ADC) with a novel DNA-alkylating effector molecule, induces DNA damage, cell cycle arrest, and apoptosis in AML cells [abstract]. Blood 2015; 126: 1366.
Stein AS, Walter RB, Erba HP, Fathi AT, Advani AS, Lancet JE et al. A phase 1 trial of SGN-CD33A as monotherapy in patients with CD33-positive acute myeloid leukemia (AML) [abstract]. Blood 2015; 126: 324.
Bixby DL, Stein AS, Fathi AT, Kovacsovics TJ, Levy MY, Erba HP et al. Vadastuximab talirine monotherapy in older patients with treatment naïve CD33-positive acute myeloid leukemia (AML) [abstract]. Blood 2016; 128: 590.
Yang J, Ravandi F, Advani AS, Vasu S, Walter RB, Faderl S et al. A phase 1b study of vadastuximab talirine as maintenance and in combination with standard consolidation for patients with acute myeloid leukemia (AML) [abstract]. Blood 2016; 128: 340.
Fathi AT, Erba HP, Lancet JE, Stein EM, Ravandi F, Faderl S et al. Vadastuximab talirine plus hypomethylating agents: a well-tolerated regimen with high remission rate in frontline older patients with acute myeloid leukemia (AML) [abstract]. Blood 2016; 128: 591.
Laszlo GS, Gudgeon CJ, Harrington KH, Dell'Aringa J, Newhall KJ, Means GD et al. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood 2014; 123: 554–561.
Reusch U, Harrington KH, Gudgeon CJ, Fucek I, Ellwanger K, Weichel M et al. Characterization of CD33/CD3 tetravalent bispecific tandem diabodies (TandAbs) for the treatment of acute myeloid leukemia. Clin Cancer Res 2016; 22: 5829–5838.
Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia 2015; 29: 1637–1647.
Acknowledgements
We thank Julian A Simon, PhD (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) for the GO cartoon. CDG is supported by a fellowship training grant from the National Heart, Lung, and Blood Institute/National Institutes of Health (NHLBI/NIH: T32-HL007093). RBW is a Leukemia & Lymphoma Society Scholar in Clinical Research. RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
RPG is a part-time employee of Celgene Corp. RBW has received laboratory research grants and/or clinical trial support from ADC Therapeutics, Inc., Amgen Inc., Amphivena Therapeutics, Inc., Aptevo Therapeutics, Inc., Covagen AG, Seattle Genetics, Inc. and Stemline Therapeutics, Inc. has ownership interests with Amphivena Therapeutics, Inc. and is (or has been) a consultant to Amphivena Therapeutics, Inc., Covagen AG, Emergent Biosolutions, Inc. (now Aptevo Therapeutics, Inc.), Pfizer, Inc., Seattle Genetics, Inc. and Jazz Pharmaceuticals, Inc.
Rights and permissions
About this article
Cite this article
Godwin, C., Gale, R. & Walter, R. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia 31, 1855–1868 (2017). https://doi.org/10.1038/leu.2017.187
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.187
This article is cited by
-
Modelling acute myeloid leukemia (AML): What’s new? A transition from the classical to the modern
Drug Delivery and Translational Research (2023)
-
Immune checkpoint targeting antibodies hold promise for combinatorial cancer therapeutics
Clinical and Experimental Medicine (2023)
-
Update on Small Molecule Targeted Therapies for Acute Myeloid Leukemia
Current Treatment Options in Oncology (2023)
-
Identification and validation of a siglec-based and aging-related 9-gene signature for predicting prognosis in acute myeloid leukemia patients
BMC Bioinformatics (2022)
-
Caspase-2 is a mediator of apoptotic signaling in response to gemtuzumab ozogamicin in acute myeloid leukemia
Cell Death Discovery (2022)