Abstract
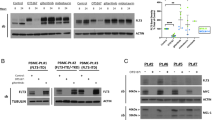
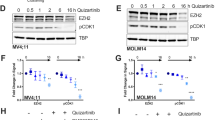
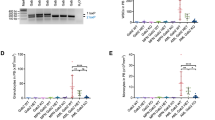
The FMS-like tyrosine kinase-3 (FLT3) gene is the most commonly mutated gene in acute myeloid leukemia (AML), and patients carrying internal tandem duplication (ITD) mutations have a poor prognosis. Long-term inhibition of FLT3 activity in these patients has been elusive. To provide a more complete understanding of FLT3 biology, a mass spectroscopy-based screen was performed to search for FLT3-interacting proteins. The screen identified dedicator of cytokinesis 2 (DOCK2), which is a guanine nucleotide exchange factor for Rho GTPases, and its expression is limited to hematolymphoid cells. We show that DOCK2 is expressed in leukemia cell lines and primary AML samples, and DOCK2 co-immunoprecipitates with wild-type FLT3 and FLT3/ITD. Knockdown (KD) of DOCK2 by shRNA selectively reduced cell proliferation and colony formation in leukemia cell lines with increased FLT3 activity, and greatly sensitized these cells to cytarabine treatment, alone and in combination with FLT3 tyrosine kinase inhibitors. DOCK2 KD in an FLT3/ITD-positive leukemia cell line also significantly prolonged survival in a mouse xenograft model. These findings suggest that DOCK2 is a potential therapeutic target for novel AML treatments, as this protein regulates the survival of leukemia cells with elevated FLT3 activity and sensitizes FLT3/ITD leukemic cells to conventional antileukemic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
McKenzie SB . Advances in understanding the biology and genetics of acute myelocytic leukemia. Clin Lab Sci 2005; 18: 28–37.
Gilliland DG, Griffin JD . The role of FLT3 in hematopoiesis and leukemia. Blood 2002; 100: 1532–1542.
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10: 1911–1918.
Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 2001; 97: 2434–2439.
Moreno I, Martin G, Bolufer P, Barragán E, Rueda E, Román J et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica 2003; 88: 19–24.
Levis M, Small D . ITDoes matter in leukemia. Leukemia 2003; 17: 1738–1752.
Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 2012; 485: 260–263.
Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 2004; 103: 3669–3676.
Levis M, Small D . FLT3 tyrosine kinase inhibitors. Int J Hematol 2005; 82: 100–107.
Gadea G, Blangy A . Dock-family exchange factors in cell migration and disease. Eur J Cell Biol 2014; 93: 466–477.
Etienne-Manneville S, Hall A . Rho GTPases in cell biology. Nature 2002; 420: 629–635.
Cancelas JA, Jansen M, Williams DA . The role of chemokine activation of Rac GTPases in hematopoietic stem cell marrow homing, retention, and peripheral mobilization. Exp Hematol 2006; 34: 976–985.
Sallmyr A, Fan J, Datta K, Kim KT, Grosu D, Shapiro P et al. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood 2008; 111: 3173–3182.
Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 2001; 412: 826–831.
Nishikimi A, Kukimoto-Niino M, Yokoyama S, Fukui Y . Immune regulatory functions of DOCK family proteins in health and disease. Exp Cell Res 2013; 319: 2343–2349.
Garcia-Bernal D, Sotillo-Mallo E, Nombela-Arrieta C, Samaniego R, Fukui Y, Stein JV et al. DOCK2 is required for chemokine-promoted human T lymphocyte adhesion under shear stress mediated by the integrin alpha4beta1. J Immunol 2006; 177: 5215–5225.
Kikuchi T, Kubonishi S, Shibakura M, Namba N, Matsui T, Fukui Y et al. Dock2 participates in bone marrow lympho-hematopoiesis. Biochem Biophys Res Commun 2008; 367: 90–96.
Wen Y, Elliott MJ, Huang Y, Miller TO, Corbin DR, Hussain LR et al. DOCK2 is critical for CD8(+) TCR(-) graft facilitating cells to enhance engraftment of hematopoietic stem and progenitor cells. Stem Cells 2014; 32: 2732–2743.
Tse KF, Mukherjee G, Small D . Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia 2000; 14: 1766–1776.
Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia 1997; 11: 1469–1477.
Zheng R, Bailey E, Nguyen B, Yang X, Piloto O, Levis M et al. Further activation of FLT3 mutants by FLT3 ligand. Oncogene 2011; 30: 4004–4014.
Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol 1989; 140: 323–334.
Lozzio CB, Lozzio BB . Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 1975; 45: 321–334.
Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst 2008; 100: 184–198.
Tkachuk DC, Kohler S, Cleary ML . Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocation in acute leukemias. Cell 1992; 71: 691–700.
Greil J, Gramatzki M, Burger R, Marschalek R, Peltner M, Trautmann U et al. The acute lymphoblastic leukaemia cell line SEM with t(4;11) chromosomal rearrangement is biphenotypic and responsive to interleukin-7. Br J Haematol 1994; 86: 275–283.
Lange B, Valtieri M, Santoli D, Caracciolo D, Mavilio F, Gemperlein I et al. Growth factor requirements of childhood acute leukemia: establishment of GM-CSF-dependent cell lines. Blood 1987; 70: 192–199.
Rosenfeld C, Goutner A, Venuat AM, Choquet C, Pico JL, Dore JF et al. An effect human leukaemic cell line: Reh. Eur J Cancer 1977; 13: 377–379.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474.
Alan JK, Lundquist EA . Mutationally activated Rho GTPases in cancer. Small GTPases 2013; 4: 159–163.
Ridley AJ . Rho proteins and cancer. Breast Cancer Res Treat 2004; 84: 13–19.
Fritz G, Just I, Kaina B . Rho GTPases are over-expressed in human tumors. Int J Cancer 1999; 81: 682–687.
Lin Y, Zheng Y . Approaches of targeting Rho GTPases in cancer drug discovery. Expert Opin Drug Discov 2015; 10: 991–1010.
Bid HK, Roberts RD, Manchanda PK, Houghton PJ . RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther 2013; 12: 1925–1934.
Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y . Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 2004; 101: 7618–7623.
Nishikimi A, Uruno T, Duan X, Cao Q, Okamura Y, Saitoh T et al. Blockade of inflammatory responses by a small-molecule inhibitor of the Rac activator DOCK2. Chem Biol 2012; 19: 488–497.
Watanabe M, Terasawa M, Miyano K, Yanagihara T, Uruno T, Sanematsu F et al. DOCK2 and DOCK5 act additively in neutrophils to regulate chemotaxis, superoxide production, and extracellular trap formation. J Immunol 2014; 193: 5660–5667.
Dobbs K, Domínguez Conde C, Zhang SY, Parolini S, Audry M, Chou J et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N Engl J Med 2015; 372: 2409–2422.
Jiang H, Pan F, Erickson LM, Jang MS, Sanui T, Kunisaki Y et al. Deletion of DOCK2, a regulator of the actin cytoskeleton in lymphocytes, suppress cardiac allograft rejection. J Exp Med 2005; 202: 1121–1130.
Yasuda K, Nundel K, Watkins AA, Dhawan T, Bonegio RG, Ubellacker JM et al. Phenotype and function of B cells and dendritic cells from interferon regulatory factor 5-deficient mice with and without a mutation in DOCK2. Int Immunol 2013; 25: 295–306.
Wang L, Nishihara H, Kimura T, Kato Y, Tanino M, Nishio M et al. DOCK2 regulates cell proliferation through Rac and ERK activation in B cell lymphoma. Biochem Biophys Res Commun 2010; 395: 111–115.
Yu J, Wu WK, Li X, He J, Li XX, Ng SS et al. Novel recurrently mutated genes and a prognostic mutation signature in colorectal cancer. Gut 2015; 64: 636–645.
Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 2013; 45: 478–486.
Lim Y, Gondek L, Li L, Wang Q, Ma H, Chang E et al. Integration of Hedgehog and mutant FLT3 signaling in myeloid leukemia. Sci Transl Med 2015; 7: 291ra96.
Choudhary C, Brandts C, Schwable J, Tickenbrock L, Sargin B, Ueker A et al. Activation mechanisms of STAT5 by oncogenic Flt3-ITD. Blood 2007; 110: 370–374.
Chatterjee A, Ghosh J, Ramdas B, Mali RS, Martin H, Kobayashi M et al. Regulation of Stat5 by FAK and PAK1 in oncogenic FLT3- and KIT-driven leukemogenesis. Cell Rep 2014; 9: 1333–1348.
Selvakumar B, Hess DT, Goldschmidt-Clermont PJ, Stamler JS . Co-regulation of constitutive nitric oxide synthases and NADPH oxidase by the small GTPase Rac. FEBS Lett 2008; 582: 2195–2202.
Espinha G, Osaki JH, Magalhaes YT, Forti FL . Rac1 GTPase-deficient HeLa cells present reduced DNA repair, proliferation, and survival under UV or gamma irradiation. Mol Cell Biochem 2015; 404: 281–297.
Canclas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA . Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med 2005; 11: 886–891.
Acknowledgements
This work was supported by grants from the National Cancer Institute (R21CA175667 to AD and R01CA90668 to DS), and a Clinician Scientist Career Award from Johns Hopkins University (to AD). DS is also supported by the Kyle Haydock Professorship. We thank Dr Alan Tackett for expert assistance with mass spectrometry experiments and data analysis.
Author contributions
MW performed the experiments, analyzed the results and wrote the manuscript; MH performed the experiments, analyzed the results and revised the manuscript; LL performed the experiments and revised the manuscript; DS analyzed the results and revised the manuscript; AD designed the study, performed the experiments, analyzed the results and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Wu, M., Hamaker, M., Li, L. et al. DOCK2 interacts with FLT3 and modulates the survival of FLT3-expressing leukemia cells. Leukemia 31, 688–696 (2017). https://doi.org/10.1038/leu.2016.284
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.284
This article is cited by
-
Requirement of splicing factor hnRNP A2B1 for tumorigenesis of melanoma stem cells
Stem Cell Research & Therapy (2021)
-
FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation
Signal Transduction and Targeted Therapy (2021)