Abstract
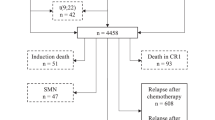
To determine the clinical significance of minimal residual disease (MRD) in patients with prognostically relevant subtypes of childhood acute lymphoblastic leukemia (ALL), we analyzed data from 488 patients treated in St Jude Total Therapy Study XV with treatment intensity based mainly on MRD levels measured during remission induction. MRD levels on day 19 predicted treatment outcome for patients with hyperdiploid >50 ALL, National Cancer Institute (NCI) standard-risk B-ALL or T-cell ALL, while MRD levels on day 46 were prognostic for patients with NCI standard-risk or high-risk B-ALL. Patients with t(12;21)/(ETV6-RUNX1) or hyperdiploidy >50 ALL had the best prognosis; those with a negative MRD on day 19 had a particularly low risk of relapse: 1.9% and 3.8%, respectively. Patients with NCI high-risk B-ALL or T-cell ALL had an inferior outcome; even with undetectable MRD on day 46, cumulative risk of relapse was 12.7% and 15.5%, respectively. Among patients with NCI standard-risk B-ALL, the outcome was intermediate overall but was poor if MRD was ⩾1% on day 19 or MRD was detectable at any level on day 46. Our results indicate that the clinical impact of MRD on treatment outcome in childhood ALL varies considerably according to leukemia subtype and time of measurement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Campana D . Minimal residual disease monitoring in childhood acute lymphoblastic leukemia. Curr Opin Hematol 2012; 19: 313–318.
Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grümayer R, Möricke A et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood 2011; 118: 2077–2084.
Bowman WP, Larsen EL, Devidas M, Linda SB, Blach L, Carroll AJ et al. Augmented therapy improves outcome for pediatric high risk acute lymphocytic leukemia: results of Children's Oncology Group trial P9906. Pediatr Blood Cancer 2011; 57: 569–577.
Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grümayer R, Möricke A et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010; 115: 3206–3214.
Stow P, Key L, Chen X, Pan Q, Neale GA, Coustan-Smith E et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood 2010; 115: 4657–4663.
Basso G, Veltroni M, Valsecchi MG, Dworzak MN, Ratei R, Silvestri D et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol 2009; 27: 5168–5174.
Sutton R, Venn NC, Tolisano J, Bahar AY, Giles JE, Ashton LJ et al. Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Br J Haematol 2009; 146: 292–299.
Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood 2008; 111: 5477–5485.
Cavé H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer—-Childhood Leukemia Cooperative Group. N Engl J Med 1998; 339: 591–598.
van Dongen JJ, Seriu T, Panzer-Grümayer ER, Biondi A, Pongers-Willemse MJ, Corral L et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 1998; 352: 1731–1738.
Coustan-Smith E, Behm FG, Sanchez J, Boyett JM, Hancock ML, Raimondi SC et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet 1998; 351: 550–554.
Ribera JM, Oriol A, Morgades M, Montesinos P, Sarrà J, González-Campos J et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol 2014; 32: 1595–1604.
Beldjord K, Chevret S, Asnafi V, Huguet F, Boulland ML, Leguay T et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood 2014; 123: 3739–3749.
Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009; 113: 4153–4162.
Paganin M, Fabbri G, Conter V, Barisone E, Polato K, Cazzaniga G et al. Postinduction minimal residual disease monitoring by polymerase chain reaction in children with acute lymphoblastic leukemia. J Clin Oncol 2014; 32: 3553–3558.
Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Houghet R et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol 2013; 14: 199–209.
Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol 2014; 15: 809–818.
Yeoh AE, Ariffin H, Chai EL, Kwok CS, Chan YH, Ponnudurai K et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol 2012; 30: 2384–2392.
Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol 2015; 16: 465–474.
Attarbaschi A, Panzer-Grümayer R, Mann G, Möricke A, König M, Mecklenbräuker A et al. Minimal residual disease-based treatment is adequate for relapse-prone childhood acute lymphoblastic leukemia with an intrachromosomal amplification of chromosome 21: the experience of the ALL-BFM 2000 trial. Klin Padiatr 2014; 226: 338–343.
Roberts KG, Pei D, Campana D, Payne-Turner D, Li Y, Cheng C et al. Outcomes of children with BCR-ABL1-like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol 2014; 32: 3012–3020.
Mullighan CG, Jeha S, Pei D, Payne-Turner D, Coustan-Smith E, Roberts KG et al. Outcome of children with hypodiploid ALL treated with risk-directed therapy based on MRD levels. Blood 2015; 126: 2896–2899.
Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009; 360: 2730–2741.
Pui CH, Evans WE . Acute lymphoblastic leuikemia. N Engl J Med 1998; 339: 605–615.
Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2011; 117: 6267–6276.
Neale GA, Coustan-Smith E, Stow P, Pan Q, Chen X, Pui CH et al. Comparative analysis of flow cytometry and polymerase chain reaction for the detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia 2004; 18: 934–938.
Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2012; 120: 5173–5180.
Kalbfleisch JD, Prentice RL . The Statistical Analysis of Failure Time Data. Wiley: New York, USA, 2002, 1–439.
Gray RJ . A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154.
Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol 2015; 33: 2938–2948.
Vora A, Andreano A, Pui CH, Hunger SP, Schrappe M, Moericke A et al. Influence of cranial radiotherapy on outcome in children with acute lymphoblastic leukemia treated with contemporary therapy. J Clin Oncol 2016; 34: 919–926.
Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 2012; 366: 1371–1381.
Bartram J, Wade R, Vora A, Hancock J, Mitchell C, Kinsey S et al. Excellent outcome of minimal residual disease-defined low-risk patients is sustained with more than 10 years follow-up: results of UK paediatric acute lymphoblastic leukaemia trials 1997-2003. Arch Dis Child 2016; 101: 449–454.
Borowitz MJ, Wood BL, Devidas M, Loh ML, Raetz EA, Salzer WL et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood 2015; 126: 964–971.
Wu D, Sherwood A, Fromm JR, Winter SS, Dunsmore KP, Loh ML et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med 2012; 4: 134ra63.
Jeha S, Pei D, Raimondi SC, Onciu M, Campana D, Cheng C et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia 2009; 23: 1406–9.
Acknowledgements
This work was supported by grants (CA21765, CA36401, and GM115279) from the National Institutes of Health and by the American Lebanese Syrian Associated Charities.
Author contributions
C-HP and DC designed the study, reviewed and interpreted data and wrote the paper; C-HP, SJ, WPB, JTS, RCR, JER, HI, TAG and WHL enrolled patients and revised the manuscript; DP and CC provided statistical expertize, analyses and data interpretation; SCR performed genetic analyses and EC-S minimal residual disease determination. All authors made substantial contributions to the concept, design and conduct of the clinical trial, were involved in the writing and critical revision of the manuscript, and gave final approval of the revision to be submitted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pui, CH., Pei, D., Raimondi, S. et al. Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with Response-Adapted therapy. Leukemia 31, 333–339 (2017). https://doi.org/10.1038/leu.2016.234
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.234
This article is cited by
-
Relationship between subtype-specific minimal residual disease level and long-term prognosis in children with acute lymphoblastic leukemia
Annals of Hematology (2024)
-
Clinical application of next-generation sequencing-based monitoring of minimal residual disease in childhood acute lymphoblastic leukemia
Journal of Cancer Research and Clinical Oncology (2023)
-
The advances of E2A-PBX1 fusion in B-cell acute lymphoblastic Leukaemia
Annals of Hematology (2023)
-
JAK3 mutations and mitochondrial apoptosis resistance in T-cell acute lymphoblastic leukemia
Leukemia (2022)
-
Low incidence of ABL-class and JAK-STAT signaling pathway alterations in uniformly treated pediatric and adult B-cell acute lymphoblastic leukemia patients using MRD risk-directed approach – a population-based study
BMC Cancer (2021)