Abstract
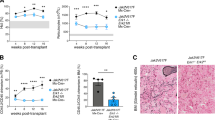
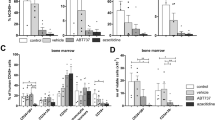
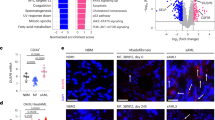
Chronic and juvenile myelomonocytic leukemias (CMML and JMML) are myelodysplastic/myeloproliferative neoplasia (MDS/MPN) overlap syndromes that respond poorly to conventional treatments. Aberrant Ras activation because of NRAS, KRAS, PTPN11, CBL and NF1 mutations is common in CMML and JMML. However, no mechanism-based treatments currently exist for cancers with any of these mutations. An alternative therapeutic strategy involves targeting Ras-regulated effector pathways that are aberrantly activated in CMML and JMML, which include the Raf/MEK/ERK and phosphoinositide-3′-OH kinase (PI3K)/Akt cascades. Mx1-Cre, KrasD12 and Mx1-Cre, Nf1flox/− mice accurately model many aspects of CMML and JMML. Treating Mx1-Cre, KrasD12 mice with GDC-0941 (also referred to as pictilisib), an orally bioavailable inhibitor of class I PI3K isoforms, reduced leukocytosis, anemia and splenomegaly while extending survival. However, GDC-0941 treatment attenuated activation of both PI3K/Akt and Raf/MEK/ERK pathways in primary hematopoietic cells, suggesting it could be acting through suppression of Raf/MEK/ERK signals. To interrogate the importance of the PI3K/Akt pathway specifically, we treated mice with the allosteric Akt inhibitor MK-2206. This compound had no effect on Raf/MEK/ERK signaling, yet it also induced robust hematologic responses in Kras and Nf1 mice with MPN. These data support investigating PI3K/Akt pathway inhibitors as a therapeutic strategy in JMML and CMML patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Emanuel PD . Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia 2008; 22: 1335–1342.
Bacher U, Haferlach T, Schnittger S, Kreipe H, Kroger N . Recent advances in diagnosis, molecular pathology and therapy of chronic myelomonocytic leukaemia. Br J Haematol 2011; 153: 149–167.
Locatelli F, Nollke P, Zecca M, Korthof E, Lanino E, Peters C et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood 2005; 105: 410–419.
Loh ML . Recent advances in the pathogenesis and treatment of juvenile myelomonocytic leukaemia. Br J Haematol 2011; 152: 677–687.
Kohlmann A, Grossmann V, Klein HU, Schindela S, Weiss T, Kazak B et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J Clin Oncol 2010; 28: 3858–3865.
Ward AF, Braun BS, Shannon KM . Targeting oncogenic Ras signaling in hematologic malignancies. Blood 2012; 120: 3397–3406.
Downward J . Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003; 3: 11–22.
Vivanco I, Sawyers CL . The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501.
Diaz-Flores E, Goldschmidt H, Depeille P, Ng V, Akutagawa J, Krisman K et al. PLC-γ and PI3K link cytokines to ERK activation in hematopoietic cells with normal and oncogenic Kras. Sci Signal 2013; 6: ra105.
Braun BS, Tuveson DA, Kong N, Le DT, Kogan SC, Rozmus J et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci USA 2004; 101: 597–602.
Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H et al. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest 2004; 113: 528–538.
Sabnis AJ, Cheung LS, Dail M, Kang HC, Santaguida M, Hermiston ML et al. Oncogenic Kras initiates leukemia in hematopoietic stem cells. PLoS Biol 2009; 7: e59.
Lyubynska N, Gorman MF, Lauchle JO, Hong WX, Akutagawa JK, Shannon K et al. A MEK inhibitor abrogates myeloproliferative disease in Kras mutant mice. Sci Transl Med 2011; 3: 76ra27.
Chang T, Krisman K, Theobald EH, Xu J, Akutagawa J, Lauchle JO et al. Sustained MEK inhibition abrogates myeloproliferative disease in Nf1 mutant mice. J Clin Invest 2013; 123: 335–339.
Liu YL, Castleberry RP, Emanuel PD . PTEN deficiency is a common defect in juvenile myelomonocytic leukemia. Leuk Res 2009; 33: 671–677.
Goodwin CB, Li XJ, Mali RS, Chan G, Kang M, Liu Z et al. PI3K p110δ uniquely promotes gain-of-function Shp2-induced GM-CSF hypersensitivity in a model of JMML. Blood 2014; 123: 2838–2842.
Gritsman K, Yuzugullu H, Von T, Yan H, Clayton L, Fritsch C et al. Hematopoiesis and RAS-driven myeloid leukemia differentially require PI3K isoform p110α. J Clin Invest 2014; 124: 1794–1809.
Yan L . Abstract #DDT01-1: MK-2206: a potent oral allosteric AKT inhibitor. Cancer Res 2009; 69 (9 Suppl): DDT01-01.
Van Meter ME, Diaz-Flores E, Archard JA, Passegue E, Irish JM, Kotecha N et al. K-RasG12D expression induces hyperproliferation and aberrant signaling in primary hematopoietic stem/progenitor cells. Blood 2007; 109: 3945–3952.
Core Team. R, R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2014.
Folkes A, Ahmadi K, Alderton W, Alix S, Baker S, Box G et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 2008; 51: 5522–5532.
Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther 2009; 8: 1725–1738.
O'Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor. GDC-0941 in breast cancer preclinical models. Clin Cancer Res 2010; 16: 3670–3683.
Wallin JJ, Guan J, Prior WW, Lee LB, Berry L, Belmont LD et al. GDC-0941, a novel class I selective PI3K inhibitor, enhances the efficacy of docetaxel in human breast cancer models by increasing cell death in vitro and in vivo. Clin Cancer Res 2012; 18: 3901–3911.
Spoerke JM, O'Brien C, Huw L, Koeppen H, Fridlyand J, Brachmann RK et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res 2012; 18: 6771–6783.
Dail M, Wong J, Lawrence J, O'Connor D, Nakitandwe J, Chen SC et al. Loss of oncogenic Notch1 with resistance to a PI3K inhibitor in T-cell leukaemia. Nature 2014; 513: 512–516.
Braun BS, Archard JA, Van Ziffle JA, Tuveson DA, Jacks TE, Shannon K . Somatic activation of a conditional KrasG12D allele causes ineffective erythropoiesis in vivo. Blood 2006; 108: 2041–2044.
Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 2007; 1: 428–442.
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B . The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 2010; 11: 329–341.
Wu WI, Voegtli WC, Sturgis HL, Dizon FP, Vigers GP, Brandhuber BJ . Crystal structure of human AKT1 with an allosteric inhibitor reveals a new mode of kinase inhibition. PLoS One 2010; 5: e12913.
Rehan M, Beg MA, Parveen S, Damanhouri GA, Zaher GF . Computational insights into the inhibitory mechanism of human AKT1 by an orally active inhibitor, MK-2206. PLoS One 2014; 9: e109705.
Le DT, Kong N, Zhu Y, Lauchle JO, Aiyigari A, Braun BS et al. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood 2004; 103: 4243–4250.
Matsuguchi T, Kraft AS . Regulation of myeloid cell growth by distinct effectors of Ras. Oncogene 1998; 17: 2701–2709.
Shieh A, Ward AF, Donlan KL, Harding-Theobald ER, Xu J, Mullighan CG et al. Defective K-Ras oncoproteins overcome impaired effector activation to initiate leukemia in vivo. Blood 2013; 121: 4884–4893.
Staffas A, Karlsson C, Persson M, Palmqvist L, Bergo MO . Wild-type KRAS inhibits oncogenic KRAS-induced T-ALL in mice. Leukemia 2015; 29: 1032–1040.
Zhang J, Socolovsky M, Gross AW, Lodish HF . Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood 2003; 102: 3938–3946.
Zhang J, Lodish HF . Constitutive activation of the MEK/ERK pathway mediates all effects of oncogenic H-ras expression in primary erythroid progenitors. Blood 2004; 104: 1679–1687.
Tang P, Gao C, Li A, Aster J, Sun L, Chai L . Differential roles of Kras and Pten in murine leukemogenesis. Leukemia 2013; 27: 1210–1214.
Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Cappellini A, Ognibene A et al. The emerging role of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogenesis. Biochim Biophys Acta 2010; 1803: 991–1002.
Elso CM, Roberts LJ, Smyth GK, Thomson RJ, Baldwin TM, Foote SJ et al. Leishmaniasis host response loci (lmr1-3) modify disease severity through a Th1/Th2-independent pathway. Genes Immun 2004; 5: 93–100.
Smyth G, Thompson R, Satterley K . Available at: http://bioinf.wehi.edu.au/software/compareCurves/ (last accessed 17 December 2013).
Acknowledgements
This work was supported by the NF Preclinical Consortium and NF Therapeutic Consortium of the Children's Tumor Foundation, and by the National Cancer Institute Cancer Center Support Grant P30CA082103. BSB was a St Baldrick’s Foundation Scholar and received support for this work from NIH awards U54CA143874 and R01CA173085, an ASH Bridge Grant from the American Society of Hematology and the Frank A Campini Foundation. TQH was supported by NIH training grants T32CA128583 and T32HD044331 and CLC was supported by T32CA108462. TC is a St Baldrick’s Foundation Fellow. MD was supported by grants from the William Lawrence and Blanche Hughes Foundation and NIH award K99CA157950.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DS and LF are employed by Genentech; MD and TC are currently employed by Genentech, but were not when contributing to this work.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Akutagawa, J., Huang, T., Epstein, I. et al. Targeting the PI3K/Akt pathway in murine MDS/MPN driven by hyperactive Ras. Leukemia 30, 1335–1343 (2016). https://doi.org/10.1038/leu.2016.14
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.14
This article is cited by
-
Oncogenic RAS promotes leukemic transformation of CUX1-deficient cells
Oncogene (2023)
-
EZH2 inactivation in RAS-driven myeloid neoplasms hyperactivates RAS-signaling and increases MEK inhibitor sensitivity
Leukemia (2021)
-
Mutations in chronic myelomonocytic leukemia and their prognostic relevance
Clinical and Translational Oncology (2021)
-
Pediatric Neoplasms Presenting with Monocytosis
Current Hematologic Malignancy Reports (2021)
-
Mutation-specific signaling profiles and kinase inhibitor sensitivities of juvenile myelomonocytic leukemia revealed by induced pluripotent stem cells
Leukemia (2019)