Abstract
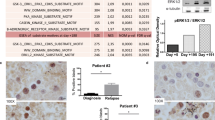
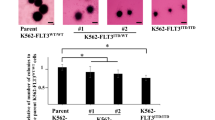
In acute myeloid leukemia (AML), about 25–30% of patients harbor a constitutively active receptor tyrosine kinase (RTK) FLT3 encoded by a FLT3 allele harboring internal tandem duplication (FLT3-ITD) mutation. The presence of FLT3-ITD correlates with poor prognosis in AML and it makes FLT3 an attractive therapeutic target in AML. Unfortunately, to date small-molecule inhibitors of FLT3 have resulted in only partial and transient clinical responses with residual leukemic blasts resistant to FLT3 inhibitors detected in blood or bone marrow. In this study, we investigated whether the RTK Axl is responsible for resistance of FLT3-ITD+ AML cells to PKC412 and AC220, FLT3 inhibitors currently under clinical trials for FLT3-ITD+ AML patients. Upon treatment with PKC412 or AC220, phosphorylation of Axl was significantly enhanced in the FLT3-ITD+ MV4-11 AML cell line and in primary blasts from a FLT3-ITD+ AML patient. Consistently, a PKC412-resistant AML cell line and PKC412-resistant primary blasts from FLT3-ITD+ AML patients had significantly higher levels of constitutively phosphorylated Axl and total Axl when compared with a PKC412-sensitive AML cell line and PKC412-sensitive primary blasts from FLT3-ITD+ AML patients. We also found that resistance of AML cells against the FLT3 inhibitor PKC412 and AC220 was substantially diminished by the inhibition of Axl via a small-molecule inhibitor TP-0903, a soluble receptor Axl fusion protein Axl-Fc or knockdown of Axl gene expression by shRNA. Collectively, our study suggests that Axl is required for resistance of FLT3-ITD+ AML cells against the FLT3 inhibitor PKC412 and AC220, and that inhibition of Axl activation may overcome resistance to FLT3-targeted therapy in FLT3-ITD+ AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brown P, Small D . FLT3 inhibitors: a paradigm for the development of targeted therapeutics for paediatric cancer. Eur J Cancer 2004; 40: 707–721, discussion; 722–724.
Knapper S . FLT3 inhibition in acute myeloid leukaemia. Br J Haematol 2007; 138: 687–699.
Stirewalt DL, Radich JP . The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 2003; 3: 650–665.
Gilliland DG, Griffin JD . The roles of FLT3 in hematopoiesis and leukemia. Blood 2002; 100: 1532–1542.
Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res 2001; 61: 7233–7239.
Eder JP, Shapiro GI, Appleman LJ, Zhu AX, Miles D, Keer H et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res 2010; 16: 3507–3516.
Kindler T, Lipka DB, Fischer T . FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 2010; 116: 5089–5102.
Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD . FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat 2009; 12: 81–89.
Hafizi S, Dahlback B . Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J 2006; 273: 5231–5244.
Hafizi S, Dahlback B . Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev 2006; 17: 295–304.
Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 1996; 271: 30022–30027.
Shankar SL, O'Guin K, Kim M, Varnum B, Lemke G, Brosnan CF et al. Gas6/Axl signaling activates the phosphatidylinositol 3-kinase/Akt1 survival pathway to protect oligodendrocytes from tumor necrosis factor alpha-induced apoptosis. J Neurosci 2006; 26: 5638–5648.
Park IK, Trotta R, Yu J, Caligiuri MA . The Axl/Gas6 pathway positively regulates FLT3 activation in human natural killer cell development. Eur J Immunol 2013; 43: 2750–2755.
Park IK, Giovenzana C, Hughes TL, Yu JH, Trotta R, Caligiuri MA . The Axl/Gas6 pathway is required for optimal cytokine signaling during human natural killer cell development. Blood 2009; 113: 2470–2477.
Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 1999; 398: 723–728.
Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G . TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 2007; 131: 1124–1136.
Avilla E, Guarino V, Visciano C, Liotti F, Svelto M, Krishnamoorthy G et al. Activation of TYRO3/AXL tyrosine kinase receptors in thyroid cancer. Cancer Res 2011; 71: 1792–1804.
Mackiewicz M, Huppi K, Pitt JJ, Dorsey TH, Ambs S, Caplen NJ . Identification of the receptor tyrosine kinase AXL in breast cancer as a target for the human miR-34a microRNA. Breast Cancer Res Treat 2011; 130: 663–679.
Neubauer A, Fiebeler A, Graham DK, O'Bryan JP, Schmidt CA, Barckow P et al. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood 1994; 84: 1931–1941.
Park IK, Mishra A, Chandler J, Whitman SP, Marcucci G, Caligiuri MA . Inhibition of the receptor tyrosine kinase Axl impedes activation of the FLT3 internal tandem duplication in human acute myeloid leukemia: implications for Axl as a potential therapeutic target. Blood 2013; 121: 2064–2073.
Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J et al. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res 2010; 70: 7570–7579.
Sun WS, Fujimoto J, Tamaya T . Coexpression of growth arrest-specific gene 6 and receptor tyrosine kinases Axl and Sky in human uterine endometrial cancers. Ann Oncol 2003; 14: 898–906.
Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene 2007; 26: 3909–3919.
Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res 2009; 69: 6871–6878.
Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012; 44: 852–860.
Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013; 497: 108–112.
Weisberg E, Ray A, Nelson E, Adamia S, Barrett R, Sattler M et al. Reversible resistance induced by FLT3 inhibition: a novel resistance mechanism in mutant FLT3-expressing cells. PLoS One 2011; 6: e25351.
Santos A, Sarmento-Ribeiro AB, de Lima MC, Simoes S, Moreira JN . Simultaneous evaluation of viability and Bcl-2 in small-cell lung cancer. Cytometry A 2008; 73A: 1165–1172.
O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 2003; 101: 3597–3605.
Ben-Batalla I, Schultze A, Wroblewski M, Erdmann R, Heuser M, Waizenegger JS et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood 2013; 122: 2443–2452.
Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT et al. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett 2008; 268: 314–324.
Zorko NA, Bernot KM, Whitman SP, Siebenaler RF, Ahmed EH, Marcucci GG et al. Mll partial tandem duplication and Flt3 internal tandem duplication in a double knock-in mouse recapitulates features of counterpart human acute myeloid leukemias. Blood 2012; 120: 1130–1136.
Meyer AS, Miller MA, Gertler FB, Lauffenburger DA . The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci Signal 2013; 6: ra66.
Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res 2013; 19: 279–290.
Dufies M, Jacquel A, Belhacene N, Robert G, Cluzeau T, Luciano F et al. Mechanisms of AXL overexpression and function in Imatinib-resistant chronic myeloid leukemia cells. Oncotarget 2011; 2: 874–885.
Zhang JM, Yang PL, Gray NS . Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 2009; 9: 28–39.
Whitman SP, Kohlschmidt J, Maharry K, Volinia S, Mrozek K, Nicolet D et al. GAS6 expression identifies high-risk adult AML patients: potential implications for therapy. Leukemia 2014; 28: 1252–1258.
Murray MY, Rushworth SA, MacEwan DJ . Micro RNAs as a new therapeutic target towards leukaemia signalling. Cell Signal 2012; 24: 363–368.
Zauli G, Voltan R, di Iasio MG, Bosco R, Melloni E, Sana ME et al. miR-34a induces the downregulation of both E2F1 and B-Myb oncogenes in leukemic cells. Clin Cancer Res 2011; 17: 2712–2724.
Choueiri TK, Vaishampayan U, Rosenberg JE, Logan TF, Harzstark AL, Bukowski RM et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol 2013; 31: 181–186.
Shah MA, Wainberg ZA, Catenacci DV, Hochster HS, Ford J, Kunz P et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One 2013; 8: e54014.
Sheridan C . First Axl inhibitor enters clinical trials. Nat Biotechnol 2013; 31: 775–776.
Korshunov VA . Axl-dependent signalling: a clinical update. Clin Sci (Lond) 2012; 122: 361–368.
Acknowledgements
We thank the OSUCCC Leukemia Tissue Bank Shared Resource for providing AML patient samples. We also acknowledge Tolero Pharmaceuticals for providing the Axl inhibitor TP-0903. This work was supported by National Cancer Institute grants (CA16058 and CA89341 to MAC) and P50 SPORE grant (CA140158 to GM and MAC). I-KP and BM-B were supported by NCI T32 training grants (CA933835).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
SLW and DJB are employees of and shareholders in Tolero Pharmaceuticals, Inc. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Park, IK., Mundy-Bosse, B., Whitman, S. et al. Receptor tyrosine kinase Axl is required for resistance of leukemic cells to FLT3-targeted therapy in acute myeloid leukemia. Leukemia 29, 2382–2389 (2015). https://doi.org/10.1038/leu.2015.147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.147
This article is cited by
-
TP-0184 inhibits FLT3/ACVR1 to overcome FLT3 inhibitor resistance and hinder AML growth synergistically with venetoclax
Leukemia (2024)
-
Sitravatinib as a potent FLT3 inhibitor can overcome gilteritinib resistance in acute myeloid leukemia
Biomarker Research (2023)
-
Indole-based FLT3 inhibitors and related scaffolds as potential therapeutic agents for acute myeloid leukemia
BMC Chemistry (2023)
-
Therapeutic targeting of the functionally elusive TAM receptor family
Nature Reviews Drug Discovery (2023)
-
Resistance to targeted therapies in acute myeloid leukemia
Clinical & Experimental Metastasis (2023)