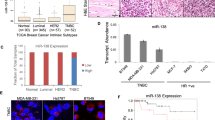
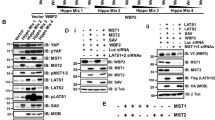
Abstract
The contribution of microRNAs to lymphoma biology is not fully understood. In particular, it remains untested whether microRNA dysregulation could contribute to the emergence of the aggressive subset of B-cell lymphomas that coexpress MYC and BCL2. Here, we identify microRNA-124 (miR-124) as a negative regulator of MYC and BCL2 expression in B-cell lymphomas. Concordantly, stable or transient ectopic expression of miR-124 suppressed cell proliferation and survival, whereas genetic inhibition of this miRNA enhanced the fitness of these tumors. Mechanistically, the activities of miR-124 towards MYC and BCL2 intersect with both oncogenic and tumor-suppressive pathways. In respect to the former, we show that miR-124 directly targets nuclear factor-κB (NF-κB) p65, and using genetic approaches, we demonstrate that this interaction accounts for the miR-124-mediated suppression of MYC and BCL2. We also characterized miR-124 promoter region and identified a functional p53 binding site. In agreement with this finding, endogenous or ectopic expression of wild-type, but not mutant, p53 increased miR-124 levels and suppressed p65, MYC and BCL2. Our data unveil an miRNA-dependent regulatory circuitry that links p53 to the NF-κB pathway, which when disrupted in B-cell lymphoma may be associated with aberrant coexpression of MYC and BCL2 and poor prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Di Lisio L, Martinez N, Montes-Moreno S, Piris-Villaespesa M, Sanchez-Beato M, Piris MA . The role of miRNAs in the pathogenesis and diagnosis of B-cell lymphomas. Blood 2012; 120: 1782–1790.
Lenz G, Staudt LM . Aggressive lymphomas. N Engl J Med 2010; 362: 1417–1429.
Abramson JS, Shipp MA . Advances in the biology and therapy of diffuse large B-cell lymphoma: moving toward a molecularly targeted approach. Blood 2005; 106: 1164–1174.
Reeder CB, Ansell SM . Novel therapeutic agents for B-cell lymphoma: developing rational combinations. Blood 2011; 117: 1453–1462.
Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ et al. Double-hit B-cell lymphomas. Blood 2011; 117: 2319–2331.
Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012; 30: 3460–3467.
Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012; 30: 3452–3459.
Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013; 121: 4021–4031, quiz 4250.
Li C, Kim SW, Rai D, Bolla AR, Adhvaryu S, Kinney MC et al. Copy number abnormalities, MYC activity, and the genetic fingerprint of normal B cells mechanistically define the microRNA profile of diffuse large B-cell lymphoma. Blood 2009; 113: 6681–6690.
An L, Liu Y, Wu A, Guan Y . MicroRNA-124 inhibits migration and invasion by down-regulating ROCK1 in glioma. PLoS One 2013; 8: e69478.
Lu Y, Yue X, Cui Y, Zhang J, Wang K . MicroRNA-124 suppresses growth of human hepatocellular carcinoma by targeting STAT3. Biochem Biophys Res Commun 2013; 441: 873–879.
Zhang J, Lu Y, Yue X, Li H, Luo X, Wang Y et al. MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PLoS One 2013; 8: e70300.
Wong KY, So CC, Loong F, Chung LP, Lam WW, Liang R et al. Epigenetic inactivation of the miR-124-1 in haematological malignancies. PLoS One 2011; 6: e19027.
Ning BF, Ding J, Liu J, Yin C, Xu WP, Cong WM et al. Hepatocyte nuclear factor 4alpha-nuclear factor-kappaB feedback circuit modulates liver cancer progression. Hepatology 2014; 60: 1607–1619.
Reinhardt HC, Schumacher B . The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends Genet 2012; 28: 128–136.
Hermeking H . MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer 2012; 12: 613–626.
Asuthkar S, Stepanova V, Lebedeva T, Holterman AL, Estes N, Cines DB et al. Multifunctional roles of urokinase plasminogen activator (uPA) in cancer stemness and chemoresistance of pancreatic cancer. Mol Biol Cell 2013; 24: 2620–2632.
Liu X, Li F, Zhao S, Luo Y, Kang J, Zhao H et al, MicroRNA-124-mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke 2013; 44: 1973–1980.
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie Y et al. MicroRNA-124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol Cell Biochem 2014; 392: 153–159.
Kim SW, Rai D, Aguiar RC . Gene set enrichment analysis unveils the mechanism for the phosphodiesterase 4B control of glucocorticoid response in B-cell lymphoma. Clin Cancer Res 2011; 17: 6723–6732.
Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC . MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci USA 2012; 109: 7865–7870.
Kim SW, Rai D, McKeller MR, Aguiar RC . Rational combined targeting of phosphodiesterase 4B and SYK in DLBCL. Blood 2009; 113: 6153–6160.
Ortega M, Bhatnagar H, Lin AP, Wang L, Aster JC, Sill H et al. A microRNA-mediated regulatory loop modulates NOTCH and MYC oncogenic signals in B- and T-cell malignancies. Leukemia 2014; 29: 968–976.
Ott G, Rosenwald A, Campo E . Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood 2013; 122: 3884–3891.
Tracey L, Perez-Rosado A, Artiga MJ, Camacho FI, Rodriguez A, Martinez N et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol 2005; 206: 123–134.
Duyao MP, Buckler AJ, Sonenshein GE . Interaction of an NF-kappa B-like factor with a site upstream of the c-myc promoter. Proc Natl Acad Sci USA 1990; 87: 4727–4731.
el-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B . Definition of a consensus binding site for p53. Nat Genet 1992; 1: 45–49.
Hunten S, Siemens H, Kaller M, Hermeking H . The p53/microRNA network in cancer: experimental and bioinformatics approaches. Adv Exp Med Biol 2013; 774: 77–101.
Acknowledgements
We thank Dr Kyung Lib Jang (Pusan National University) for the WT and MUT (R248Q) p53 constructs. This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013R1A1A2008838) to S-WK and RCTA was funded by NCI/NIH R01-CA138747 and Veterans Administration Merit Award (I01-BX001882).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Jeong, D., Kim, J., Nam, J. et al. MicroRNA-124 links p53 to the NF-κB pathway in B-cell lymphomas. Leukemia 29, 1868–1874 (2015). https://doi.org/10.1038/leu.2015.101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.101
This article is cited by
-
Human bone marrow-derived mesenchymal stem overexpressing microRNA-124-3p inhibit DLBCL progression by downregulating the NFATc1/cMYC pathway
Stem Cell Research & Therapy (2023)
-
NF-κB p65 represses microRNA-124 transcription in diffuse large B-cell lymphoma
Genes & Genomics (2020)
-
P53 vs NF-κB: the role of nuclear factor-kappa B in the regulation of p53 activity and vice versa
Cellular and Molecular Life Sciences (2020)
-
Disruption of the Myc-PDE4B regulatory circuitry impairs B-cell lymphoma survival
Leukemia (2019)
-
Pyrosequencing quantified methylation level of miR-124 predicts shorter survival for patients with myelodysplastic syndrome
Clinical Epigenetics (2017)