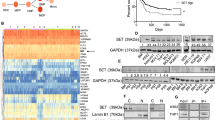
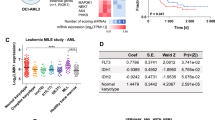
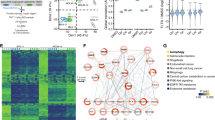
Abstract
Single kinase-targeted cancer therapies often failed prolonged responses because cancer cells bypass through alternative routes. In this study, high-throughput kinomic and proteomic approaches enabled to identify aberrant activity profiles in mixed lineage leukemia (MLL)-rearranged acute myeloid leukemia (AML) that defined druggable targets. This approach revealed impaired activity of proteins belonging to the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathway. Pharmacological druggable MAPK pathway targets tested in primary MLL-rearranged AML included MAPKK1/2 (MEK), cyclic AMP-responsive element-binding protein (CREB) and MAPK8/9 (JNK). MEK inhibition showed to severely decrease MLL-rearranged AML cell survival without showing cytotoxicity in normal controls, whereas inhibition of CREB and JNK failed to exhibit MLL selectivity. Exploring the working mechanism of MEK inhibition, we assessed proteome activity in response to MEK inhibition in THP-1. MAPK1/3 (Erk) phosphorylation was instantly decreased in concurrence with a sustained Akt/mammalian target of rapamycin (mTOR) phosphorylation that enabled a subpopulation of cells to survive MEK inhibition. After exhaustion of MEK inhibition the AML cells recovered via increased activity of vascular endothelial growth factor receptor-2 (VEGFR-2) and Erk proteins to resume their proliferative state. Combined MEK and VEGFR-2 inhibition strengthened the reduction in MLL-rearranged AML cell survival by blocking the Akt/mTOR and MAPK pathways simultaneously. The generation of insights in cancerous altered activity profiles and alternative escape mechanisms upon targeted therapy allows the rational design of novel combination strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chapuis N, Park S, Leotoing L, Tamburini J, Verdier F, Bardet V et al. IkappaB kinase overcomes PI3K/Akt and ERK/MAPK to control FOXO3a activity in acute myeloid leukemia. Blood 2010; 116: 4240–4250.
Chen E, Staudt LM, Green AR . Janus kinase deregulation in leukemia and lymphoma. Immunity 2012; 36: 529–541.
Tamburini J, Elie C, Bardet V, Chapuis N, Park S, Broet P et al. Constitutive phosphoinositide 3-kinase/Akt activation represents a favorable prognostic factor in de novo acute myelogenous leukemia patients. Blood 2007; 110: 1025–1028.
Ter Elst A, Diks SH, Kampen KR, Hoogerbrugge PM, Ruijtenbeek R, Boender PJ et al. Identification of new possible targets for leukemia treatment by kinase activity profiling. Leuk Lymphoma 2011; 52: 122–130.
Kornblau SM, Coombes KR . Use of reverse phase protein microarrays to study protein expression in leukemia: technical and methodological lessons learned. Methods Mol Biol 2011; 785: 141–155.
Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther 2006; 5: 2512–2521.
Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 2002; 346: 645–652.
Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell 2012; 149: 307–321.
Dokter WH, Tuyt L, Sierdsema SJ, Esselink MT, Vellenga E . The spontaneous expression of interleukin-1 beta and interleukin-6 is associated with spontaneous expression of AP-1 and NF-kappa B transcription factor in acute myeloblastic leukemia cells. Leukemia 1995; 9: 425–432.
Lunghi P, Tabilio A, Dall’Aglio PP, Ridolo E, Carlo-Stella C, Pelicci PG et al. Downmodulation of ERK activity inhibits the proliferation and induces the apoptosis of primary acute myelogenous leukemia blasts. Leukemia 2003; 17: 1783–1793.
Odenike O, Curran E, Iyengar N, Popplewell L, Kirschbaum M, Erba HP et al. Phase II Study of the Oral MEK Inhibitor AZD6244 in Advanced Acute Myeloid Leukemia (AML). Blood 2009; 114: 821–822.
Anselmi F, Orlandini M, Rocchigiani M, De CC, Salameh A, Lentucci C et al. c-ABL modulates MAP kinases activation downstream of VEGFR-2 signaling by direct phosphorylation of the adaptor proteins GRB2 and NCK1. Angiogenesis 2012; 15: 187–197.
Cierpicki T, Risner LE, Grembecka J, Lukasik SM, Popovic R, Omonkowska M et al. Structure of the MLL CXXC domain-DNA complex and its functional role in MLL-AF9 leukemia. Nat Struct Mol Biol 2010; 17: 62–68.
Faber J, Krivtsov AV, Stubbs MC, Wright R, Davis TN, Heuvel-Eibrink M et al. HOXA9 is required for survival in human MLL-rearranged acute leukemias. Blood 2009; 113: 2375–2385.
Liedtke M, Cleary ML . Therapeutic targeting of MLL. Blood 2009; 113: 6061–6068.
Wang Z, Iwasaki M, Ficara F, Lin C, Matheny C, Wong SH et al. GSK-3 promotes conditional association of CREB and its coactivators with MEIS1 to facilitate HOX-mediated transcription and oncogenesis. Cancer Cell 2010; 17: 597–608.
Balgobind BV, Hollink IH, Arentsen-Peters ST, Zimmermann M, Harbott J, Beverloo HB et al. Integrative analysis of type-I and type-II aberrations underscores the genetic heterogeneity of pediatric acute myeloid leukemia. Haematologica 2011; 96: 1478–1487.
Young A, Lou D, McCormick F . Oncogenic and wild-type Ras play divergent roles in the regulation of mitogen-activated protein kinase signaling. Cancer Discov 2013; 3: 112–123.
Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov 2011; 1: 248–259.
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012; 487: 505–509.
Gallay N, Dos SC, Cuzin L, Bousquet M, Simmonet GV, Chaussade C et al. The level of AKT phosphorylation on threonine 308 but not on serine 473 is associated with high-risk cytogenetics and predicts poor overall survival in acute myeloid leukaemia. Leukemia 2009; 23: 1029–1038.
Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood 2006; 108: 2358–2365.
Acknowledgements
RAS pathway mutations were obtained from MWJ Fornerod, MM van den Heuvel-Eibrink and CM Zwaan from the Department of Pediatric Oncology/Hematology, Erasmus MC—Sophia Children’s Hospital, Rotterdam, Netherlands. We thank Imclone for their generous supply of VEGFR-2 antibody IMC1121b. We also thank the patients who donated leukemia specimens and also physician assistants, nurse practitioners and fellows who acquired specimens. KRK was supported by a grant from the Foundation for Pediatric Oncology Groningen, The Netherlands (SKOG). The kinome data collection was supported by a grant from the Foundation KiKa, Amstelveen (AtE, HM and ESJMdB).
Author Contributions
KRK performed research, collected data, analyzed data and wrote the paper. AtE performed peptide array and background subtraction. HM performed statistical testing of peptide array results. SHD guided performing the peptide arrays. FJGS performed research and collected data. VdH contributed samples. MPP designed peptide arrays. VG performed quantile normalization and supervised peptide array analysis. ESJMdB designed research, analyzed data, supervised and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Kampen, K., ter Elst, A., Mahmud, H. et al. Insights in dynamic kinome reprogramming as a consequence of MEK inhibition in MLL-rearranged AML. Leukemia 28, 589–599 (2014). https://doi.org/10.1038/leu.2013.342
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.342
Keywords
This article is cited by
-
SET-PP2A complex as a new therapeutic target in KMT2A (MLL) rearranged AML
Oncogene (2023)
-
LncRNA CD27-AS1 promotes acute myeloid leukemia progression through the miR-224-5p/PBX3 signaling circuit
Cell Death & Disease (2021)
-
Targeted therapy of human leukemia xenografts in immunodeficient zebrafish
Scientific Reports (2021)
-
Drug and disease signature integration identifies synergistic combinations in glioblastoma
Nature Communications (2018)
-
Epidermal growth factor receptor is expressed and active in a subset of acute myeloid leukemia
Journal of Hematology & Oncology (2016)