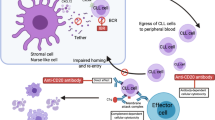
Abstract
Understanding the pathogenesis of CLL has uncovered a plethora of novel targets for human application of monoclonal antibodies, engineered T cells, or inhibitors of signal transduction pathways. The B-cell receptor signaling pathway is being actively explored as a therapeutic target in CLL. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase is showing impressive responses in heavily pre-treated high-risk CLL, whether alone or in combination with MoAbs or chemotherapy. Other key components of the BCR pathway, namely PI3K-δ, are also being targeted with novel therapies with promising results as well. Future trials would likely evaluate ibrutinib in the front-line setting. Moreover, improvements in allogeneic HCT mostly by continuing to reduce associated toxicity as well as incorporating cellular therapies such as autologous CLL tumor vaccines, among others, will continue to expand. This is also the case for the next generation of chimeric antigen receptor therapy for CLL once genetically modified T cells are available at broad scale and with improved efficacy. As our ability to further refine and integrate these therapies continues to improve, and we gain further knowledge from gene sequencing, we anticipate that treatment algorithms will continue to be revised to a more personalized approach to treat this disease with improved efficacy and devoid of unnecessary toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Foon KA, Hallek MJ . Changing paradigms in the treatment of chronic lymphocytic leukemia. Leukemia 2010; 24: 500–511.
Gribben JG, O’Brien S . Update on therapy of chronic lymphocytic leukemia. J Clin Oncol 2011; 29: 544–550.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42.
Kharfan-Dabaja MA, Fahed R, Hussein M, Santos ES . Evolving role of monoclonal antibodies in the treatment of chronic lymphocytic leukemia. Expert Opin Investig Drugs 2007; 16: 1799–1815.
Jain N, O'Brien S . Ibrutinib (PCI-32765) in chronic lymphocytic leukemia. Hematol Oncol Clin North Am 2013; 27: 851–860.
Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM Jr, Wagner-Johnston ND et al. CAL-101, An isoform-selective inhibitor of phosphatidylinositol 3-kinase P110{delta}, demonstrates clinical activity and pharmacodynamic effects in patients with relapsed or refractory chronic lymphocytic leukemia. ASH Ann Meet Abstr 2010; 116: 55.
Keating MJ, O'Brien S, Albitar M, Lerner S, Plunkett W, Giles F et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005; 23: 4079–4088.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol 2008; 26: 4912–4920.
Ritgen M, Bottcher S, Stilgenbauer S, Bunjes D, Schubert J, Cohen S et al. Quantitative MRD monitoring identifies distinct GVL response patterns after allogeneic stem cell transplantation for chronic lymphocytic leukemia: results from the GCLLSG CLL3X trial. Leukemia 2008; 22: 1377–1386.
Dreger P, Dohner H, Ritgen M, Bottcher S, Busch R, Dietrich S et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood 2010; 116: 2438–2447.
Brown JR, Kim HT, Armand P, Cutler C, Fisher DC, Ho V et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia 2013; 27: 362–369.
Kharfan-Dabaja MA, Bazarbachi A . Hematopoietic stem cell allografting for chronic lymphocytic leukemia: a focus on reduced-intensity conditioning regimens. Cancer Control 2012; 19: 68–75.
Buggy JJ, Elias L . Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol 2012; 31: 119–132.
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010; 107: 13075–13080.
Brown JR, Furman RR, Flinn I, Coutre SE, Wagner-Johnston ND, Kahl BS et al. Final results of a phase I study of idelalisib (GSE1101) a selective inhibitor of PI3K{delta}, in patients with relapsed or refractory CLL. ASCO Meeting Abstracts 2013; 31 (15_suppl): 7003.
Porter DL, Levine BL, Kalos M, Bagg A, June CH . Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733.
Giordano Attianese GM, Marin V, Hoyos V, Savoldo B, Pizzitola I, Tettamanti S et al. In vitro and in vivo model of a novel immunotherapy approach for chronic lymphocytic leukemia by anti-CD23 chimeric antigen receptor. Blood 2011; 117: 4736–4745.
Wierda W, O'Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 2005; 23: 4070–4078.
Goede V, Fischer K, Busch R, Jaeger U, Dilhuydy MS, Wickham N et al. Chemoimmunotherapy with GA101 plus chlorambucil in patients with chronic lymphocytic leukemia and comorbidity: results of the CLL11 (BO21004) safety run-in. Leukemia 2013; 27: 1172–1174.
Elter T, James R, Busch R, Winkler D, Ritgen M, Bottcher S et al. Fludarabine and cyclophosphamide in combination with alemtuzumab in patients with primary high-risk, relapsed or refractory chronic lymphocytic leukemia. Leukemia 2012; 26: 2549–2552.
Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood 2007; 109: 405–411.
Lamanna N, Kalaycio M, Maslak P, Jurcic JG, Heaney M, Brentjens R et al. Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J Clin Oncol 2006; 24: 1575–1581.
Castro JE, James DF, Sandoval-Sus JD, Jain S, Bole J, Rassenti L et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia 2009; 23: 1779–1789.
O'Brien SM, Kantarjian H, Thomas DA, Giles FJ, Freireich EJ, Cortes J et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 2001; 19: 2165–2170.
Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 2006; 177: 362–371.
Bologna L, Gotti E, Da Roit F, Intermesoli T, Rambaldi A, Introna M et al. Ofatumumab is more efficient than rituximab in lysing B chronic lymphocytic leukemia cells in whole blood and in combination with chemotherapy. J Immunol 2013; 190: 231–239.
Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol 2010; 28: 1749–1755.
Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Osterborg A . Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood 2011; 118: 5126–5129.
Wierda WG, Kipps TJ, Durig J, Griskevicius L, Stilgenbauer S, Mayer J et al. Chemoimmunotherapy with O-FC in previously untreated patients with chronic lymphocytic leukemia. Blood 2011; 117: 6450–6458.
Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 2011; 117: 4519–4529.
Patz M, Isaeva P, Forcob N, Muller B, Frenzel LP, Wendtner CM et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol 2011; 152: 295–306.
Morschhauser F, Cartron G, Lamy T, Milpied N-J, Thieblemont C, Tilly H et al. Phase I Study of RO5072759 (GA101) in Relapsed/Refractory Chronic Lymphocytic Leukemia. ASH Annual Meeting Abstracts, November 20, 2009; 114: 884.
Goede V, Fischer K, Humphrey K, Asikanius E, Busch R, Engelke A et al. Obinutuzumab (GA101) plus chlorambucil (Clb) or rituximab (R) plus Clb versus Clb alone in patients with chronic lymphocytic leukemia (CLL) and preexisting medical conditions (comorbidities): Final stage 1 results of the CLL11 (BO21004) phase III trial. ASCO Meeting Abstracts 2013; 31 (15_suppl): 7004.
Hale G, Dyer MJ, Clark MR, Phillips JM, Marcus R, Riechmann L et al. Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet 1988; 2: 1394–1399.
Treumann A, Lifely MR, Schneider P, Ferguson MA . Primary structure of CD52. J Biol Chem 1995; 270: 6088–6099.
Stanglmaier M, Reis S, Hallek M . Rituximab and alemtuzumab induce a nonclassic, caspase-independent apoptotic pathway in B-lymphoid cell lines and in chronic lymphocytic leukemia cells. Ann Hematol 2004; 83: 634–645.
Bindon CI, Hale G, Waldmann H . Importance of antigen specificity for complement-mediated lysis by monoclonal antibodies. Eur J Immunol 1988; 18: 1507–1514.
Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 2007; 25: 5616–5623.
Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002; 99: 3554–3561.
Osterborg A, Dyer MJ, Bunjes D, Pangalis GA, Bastion Y, Catovsky D et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European study group of CAMPATH-1H treatment in chronic lymphocytic leukemia. J Clin Oncol 1997; 15: 1567–1574.
Rai KR, Freter CE, Mercier RJ, Cooper MR, Mitchell BS, Stadtmauer EA et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol 2002; 20: 3891–3897.
Byrd JC, Kipps TJ, Flinn IW, Castro J, Lin TS, Wierda W et al. Phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. Blood 2010; 115: 489–495.
Krause G, Patz M, Isaeva P, Wigger M, Baki I, Vondey V et al. Action of novel CD37 antibodies on chronic lymphocytic leukemia cells. Leukemia 2012; 26: 546–549.
Law CL, Gordon KA, Collier J, Klussman K, McEarchern JA, Cerveny CG et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res 2005; 65: 8331–8338.
Lapalombella R, Gowda A, Joshi T, Mehter N, Cheney C, Lehman A et al. The humanized CD40 antibody SGN-40 demonstrates pre-clinical activity that is enhanced by lenalidomide in chronic lymphocytic leukaemia. Br J Haematol 2009; 144: 848–855.
Camacho LH, Joyce R, Brown JR, Chanan-Khan A, Amrein PC, Assad A et al. A phase 1, open-label, multi-center, multiple-dose, dose-escalation study of MDX-1342 in patients with CD19-positive refractory/relapsed chronic lymphocytic leukemia. ASH Annual Meeting Abstracts 2009; 114: 3425.
Stein R, Qu Z, Chen S, Solis D, Hansen HJ, Goldenberg DM . Characterization of a humanized IgG4 anti-HLA-DR monoclonal antibody that lacks effector cell functions but retains direct antilymphoma activity and increases the potency of rituximab. Blood 2006; 108: 2736–2744.
Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D et al. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol 2007; 178: 5595–5605.
Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest 2008; 118: 2427–2437.
Ramsay AG, Clear AJ, Fatah R, Gribben JG . Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood 2012; 120: 1412–1421.
Dustin ML, Depoil D . New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol 2011; 11: 672–684.
Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol 2011; 29: 1175–1181.
Shanafelt TD, Ramsay AG, Zent CS, Leis JF, Tun HW, Call TG et al. Long-term repair of T-cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL). Blood 2013; 121: 4137–4141.
Lapalombella R, Yu B, Triantafillou G, Liu Q, Butchar JP, Lozanski G et al. Lenalidomide down-regulates the CD20 antigen and antagonizes direct and antibody-dependent cellular cytotoxicity of rituximab on primary chronic lymphocytic leukemia cells. Blood 2008; 112: 5180–5189.
Badoux XC, Keating MJ, Wen S, Wierda WG, O'Brien SM, Faderl S et al. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 2013; 31: 584–591.
Badoux XC, Keating MJ, Wen S, Lee BN, Sivina M, Reuben J et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood 2011; 118: 3489–3498.
Gribben JG . Stem cell transplantation in chronic lymphocytic leukemia. Biol Blood Marrow Transplant 2009; 15 (1 Suppl): 53–58.
Sutton L, Chevret S, Tournilhac O, Divine M, Leblond V, Corront B et al. Autologous stem cell transplantation as a first-line treatment strategy for chronic lymphocytic leukemia: a multicenter, randomized, controlled trial from the SFGM-TC and GFLLC. Blood 2011; 117: 6109–6119.
Kharfan-Dabaja MA, Kumar A, Behera M, Djulbegovic B . Systematic review of high dose chemotherapy and autologous haematopoietic stem cell transplantation for chronic lymphocytic leukaemia: what is the published evidence? Br J Haematol 2007; 139: 234–242.
Michallet M, Archimbaud E, Bandini G, Rowlings PA, Deeg HJ, Gahrton G et al. HLA-identical sibling bone marrow transplantation in younger patients with chronic lymphocytic leukemia. European Group for Blood and Marrow Transplantation and the International Bone Marrow Transplant Registry. Ann Intern Med 1996; 124: 311–315.
Pavletic ZS, Arrowsmith ER, Bierman PJ, Goodman SA, Vose JM, Tarantolo SR et al. Outcome of allogeneic stem cell transplantation for B cell chronic lymphocytic leukemia. Bone Marrow Transplant 2000; 25: 717–722.
Toze CL, Galal A, Barnett MJ, Shepherd JD, Conneally EA, Hogge DE et al. Myeloablative allografting for chronic lymphocytic leukemia: evidence for a potent graft-versus-leukemia effect associated with graft-versus-host disease. Bone Marrow Transplant 2005; 36: 825–830.
Dreger P, Brand R, Milligan D, Corradini P, Finke J, Lambertenghi Deliliers G et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia 2005; 19: 1029–1033.
Kharfan-Dabaja MA, Pidala J, Kumar A, Terasawa T, Djulbegovic B . Comparing efficacy of reduced-toxicity allogeneic hematopoietic cell transplantation with conventional chemo-(immuno) therapy in patients with relapsed or refractory CLL: a Markov decision analysis. Bone Marrow Transplant 2012; 47: 1164–1170.
Burkhardt UE, Hainz U, Stevenson K, Goldstein NR, Pasek M, Naito M et al. Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J Clin Invest 2013; 123: 3756–3765.
El-Jurdi N, Reljic T, Kumar A, Pidala J, Bazarbachi A, Djulbegovic B et al. Efficacy of adoptive immunotherapy with donor lymphocyte infusion in relapsed lymphoid malignancies. Immunotherapy 2013; 5: 457–466.
Jena B, Dotti G, Cooper LJ . Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 2010; 116: 1035–1044.
Sadelain M, Brentjens R, Riviere I . The basic principles of chimeric antigen receptor design. Cancer Discov 2013; 3: 388–398.
Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK . A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther 2005; 12: 933–941.
Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 2012; 119: 3940–3950.
Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS ONE 2013; 8: e64138.
Huls MH, Figliola MJ, Dawson MJ, Olivares S, Kebriaei P, Shpall EJ et al. Clinical application of Sleeping Beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood. J Vis Exp 2013; 72: e50070.
Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008; 112: 2261–2271.
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra138.
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011; 3: 95ra73.
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013; e-pub ahead of print 20 September 2013 doi:10.1182/blood-2013-08-519413.
Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118: 4817–4828.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518.
Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M . Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther 2010; 18: 666–668.
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119: 2709–2720.
John LB, Devaud C, Duong CM, Yong C, Beavis PA, Haynes NM et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 2013; 19: 3153–3164.
Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res 2013; 19: 3153–3164.
Fukuda T, Chen L, Endo T, Tang L, Lu D, Castro JE et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci USA 2008; 105: 3047–3052.
Gluckman E . Hematopoietic stem-cell transplants using umbilical-cord blood. N Engl J Med 2001; 344: 1860–1861.
Weber G, Gerdemann U, Caruana I, Savoldo B, Hensel NF, Rabin KR et al. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia 2013; 27: 1538–1547.
Castro JE, Melo-Cardenas J, Urquiza M, Barajas-Gamboa JS, Pakbaz RS, Kipps TJ . Gene immunotherapy of chronic lymphocytic leukemia: a phase I study of intranodally injected adenovirus expressing a chimeric CD154 molecule. Cancer Res 2012; 72: 2937–2948.
Genevier HC, Hinshelwood S, Gaspar HB, Rigley KP, Brown D, Saeland S et al. Expression of Bruton's tyrosine kinase protein within the B cell lineage. Eur J Immunol 1994; 24: 3100–3105.
Wiestner A . Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood 2012; 120: 4684–4691.
Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011; 117: 6287–6296.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013; 369: 507–516.
Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012; 119: 1182–1189.
Burger JA, Keating MJ, Wierda WG, Hoellenriegel J, Ferrajoli A, Faderl S et al. The Btk Inhibitor ibrutinib (PCI-32765) in combination with rituximab is well tolerated and displays profound activity in high-risk chronic lymphocytic leukemia (CLL) patients. ASH Annual Meeting Abstracts 2012; 120: 187.
Jaglowski SM, Jones JA, Flynn JM, Andritsos LA, Maddocks KJ, Blum KA et al. A phase Ib/II study evaluating activity and tolerability of BTK inhibitor PCI-32765 and ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and related diseases. ASCO Meeting Abstracts 2012; 30 (15_suppl): 6508.
Brown JBJ, Flinn I, Barr P, Burger J, Navarro T . The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib combined with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL, interim results of a phase IB/II study. Haematologica 2012 17th Congress of the European Hematology Association Abstract 0543 2012.
Chang BY, Furman RR, Zapatka M, Barrientos JC, Li D, Steggerda S et al. Use of tumor genomic profiling to reveal mechanisms of resistance to the BTK inhibitor ibrutinib in chronic lymphocytic leukemia (CLL). ASCO Meeting Abstracts 2013; 31 (15_suppl): 7014.
Caballero D, Garcia-Marco JA, Martino R, Mateos V, Ribera JM, Sarra J et al. Allogeneic transplant with reduced intensity conditioning regimens may overcome the poor prognosis of B-cell chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene and chromosomal abnormalities (11q- and 17p-). Clin Cancer Res 2005; 11: 7757–7763.
Schetelig J, van Biezen A, Brand R, Caballero D, Martino R, Itala M et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for blood and marrow transplantation analysis. J Clin Oncol 2008; 26: 5094–5100.
Brown JR, Sharman JP, Harb WA, Kelly KR, Schreeder MT, Sweetenham JW et al. Phase Ib trial of AVL-292, a covalent inhibitor of Bruton’s tyrosine kinase (Btk), in chronic lymphocytic leukemia (CLL) and B-non-Hodgkin lymphoma (B-NHL). ASCO Meeting Abstracts 2012; 30 (15_suppl): 8032.
Brown JR, Harb WA, Gabrilove JL, Sharman JP, Hill BT, Ma S et al. Phase 1 study of single agent CC-292, a highly selective Bruton’s tyrosine kinase (Btk) inhibitor, in relapsed/refractory chronic lymphocytic leukemia (CLL). XViwCLL, Cologne, Germany, 2013.
Davids MS, Brown JR . Phosphoinositide 3′-kinase inhibition in chronic lymphocytic leukemia. Hematol Oncol Clin North Am 2013; 27: 329–339.
Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 2011; 118: 3603–3612.
Barrientos JC, Furman RR, Leonard J, Flinn I, Rai KR, De Vos S et al. Update on a phase I study of the selective PI3K{delta} inhibitor idelalisib (GS-1101) in combination with rituximab and/or bendamustine in patients with relapsed or refractory CLL. ASCO Meeting Abstracts 2013; 31 (15_suppl): 7017.
O'Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA et al. A phase II study of the selective phosphatidylinositol 3-kinase delta (PI3K{delta}) inhibitor idelalisib (GS-1101) in combination with rituximab (R) in treatment-naive patients (pts) >=65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). ASCO Meeting Abstracts 2013; 31 (15_suppl): 7005.
Gauld SB, Dal Porto JM, Cambier JC . B cell antigen receptor signaling: roles in cell development and disease. Science 2002; 296: 1641–1642.
Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010; 115: 2578–2585.
Johnson AJ, Yeh YY, Smith LL, Wagner AJ, Hessler J, Gupta S et al. The novel cyclin-dependent kinase inhibitor dinaciclib (SCH727965) promotes apoptosis and abrogates microenvironmental cytokine protection in chronic lymphocytic leukemia cells. Leukemia 2012; 26: 2554–2557.
Flynn JM, Jones JA, Andritsos L, Blum KA, Johnson AJ, Hessler J et al. Update on the phase I study of the cyclin dependent kinase inhibitor dinaciclib (SCH 727965) in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): confirmation of clinical activity and feasibility of long-term administration. ASH Annual Meeting Abstracts 2010; 116: 1396.
Kadia T, Delioukina ML, Kantarjian HM, Keating MJ, Wierda WG, Burger JA et al. A pilot phase II study of the lyn kinase inhibitor bafetinib in patients with relapsed or refractory B cell chronic lymphocytic leukemia. ASH Annual Meeting Abstracts 2011; 118: 2858.
Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1910–1916.
Kharfan-Dabaja MA, Chavez JC, Khorfan KA, Pinilla-Ibarz J . Clinical and therapeutic implications of the mutational status of IgVH in patients with chronic lymphocytic leukemia. Cancer 2008; 113: 897–906.
Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011; 475: 101–105.
Dohner H, Fischer K, Bentz M, Hansen K, Benner A, Cabot G et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood 1995; 85: 1580–1589.
el Rouby S, Thomas A, Costin D, Rosenberg CR, Potmesil M, Silber R et al. p53 gene mutation in B-cell chronic lymphocytic leukemia is associated with drug resistance and is independent of MDR1/MDR3 gene expression. Blood 1993; 82: 3452–3459.
Sturm I, Bosanquet AG, Hermann S, Guner D, Dorken B, Daniel PT . Mutation of p53 and consecutive selective drug resistance in B-CLL occurs as a consequence of prior DNA-damaging chemotherapy. Cell Death Differ 2003; 10: 477–484.
Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia 2007; 21: 12–17.
Hosing C, Kebriaei P, Wierda W, Jena B, Cooper LJ, Shpall E . CARs in chronic lymphocytic leukemia — ready to drive. Curr Hematol Malig Rep 2013; 8: 60–70.
Sentman CL . Challenges of creating effective chimeric antigen receptors for cancer therapy. Immunotherapy 2013; 5: 783–785.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr LJN Cooper founded and owns InCellerate Inc. He has patents with Sangamo Biosciences regarding artificial nucleases. He consults with American Stem Cells, Inc. and GE Healthcare. The remaining authors declare no relevant conflict of interest.
Rights and permissions
About this article
Cite this article
Kharfan-Dabaja, M., Wierda, W. & Cooper, L. Immunotherapy for chronic lymphocytic leukemia in the era of BTK inhibitors. Leukemia 28, 507–517 (2014). https://doi.org/10.1038/leu.2013.311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.311
Keywords
This article is cited by
-
Immunomodulation in leukemia: cellular aspects of anti-leukemic properties
Clinical and Translational Oncology (2020)
-
Improved disease markers suggest dual response in a patient with metastatic castration resistant prostate cancer and chronic lymphocytic leukemia following active cellular immunotherapy
Journal of Hematology & Oncology (2015)
-
Antileukemia multifunctionality of CD4+ T cells genetically engineered by HLA class I-restricted and WT1-specific T-cell receptor gene transfer
Leukemia (2015)
-
High-dose therapy and autologous hematopoietic cell transplantation as front-line consolidation in chronic lymphocytic leukemia: a systematic review
Bone Marrow Transplantation (2015)
-
B-cell receptor pathway inhibitors affect CD20 levels and impair antitumor activity of anti-CD20 monoclonal antibodies
Leukemia (2014)