Abstract
An unrelated donor (UD) search was submitted to the Italian Bone Marrow Donor Registry between February 2002 and December 2004, for 326 consecutive patients with hematological malignancies, eligible for a reduced intensity conditioning (RIC) UD transplant. Only two regimens were allowed: melphalan, alemtuzumab, fludarabine and total body irradiation of 200 cGy (regimen A) and thiotepa, cyclophosphamide, anti-thymocyte globulin (regimen B). The outcome of patients receiving an UD transplant (n=121) was compared with patients who did not find a donor (n=205), in a time dependent analysis, correcting for time to transplant. The median follow up from activation of donor search was 6.1 years. UD transplant was associated with a significantly better survival in patients with acute leukemia and non-Hodgkin's lymphoma (NHL) whereas only a favorable trend was documented for Hodgkin's disease. No survival benefit was registered for chronic leukemias. The outcome of the two different conditioning regimens was comparable, in terms of survival, transplant-related mortality and graft versus host disease. In conclusion, finding an UD and undergoing a RIC transplant significantly improves survival of patients with acute leukemia and NHL. The advantage is less clear for HD and chronic leukemias. The role of different conditioning regimens remains to be elucidated by prospective clinical trials.
Similar content being viewed by others
Introduction
For many patients with advanced hematological malignancies, allogeneic hematopoietic stem cells transplantation may represent an effective, potentially curative treatment modality. Unfortunately, most patients lack a human leukocyte antigen compatible family donor so that the possibility to identify an unrelated donor (UD) may be crucial. However, even when an UD is found, the clinical outcome after allogeneic transplantation may be poor for patients with medical comorbidities or advanced age or advanced disease such as those relapsed after a previous autologous transplant. Over the past years, for these patients, reduced intensity conditioning (RIC) programs have been developed and widely used and they have contributed significantly to reducing the mortality rate after allogeneic transplantation.1 Although this approach may be effective, the outcome of many patients is still unsatisfactory because the rate of relapse both in myeloid2, 3 and lymphoid malignancies4, 5 and the transplant-related mortality are still relevant. In addition, the time needed to identify a donor may be remarkably different from patient to patient.6 All in all, it is still difficult to fully appreciate the real impact on survival offered by these transplant procedures to an unselected group of patients from the start of the UD search.
For this reason, we analyzed the clinical outcome of an unselected consecutive series of patients for whom an UD search was activated between February 2002 and December 2004 with the intent to perform an allogeneic hematopoietic stem cells transplantation after a RIC regimen.
Patients and methods
Eligibility criteria
Eligible to this study were 326 consecutive patients for whom the UD search activation was recorded by the Italian Bone Marrow Donor Registry, between 1st February 2002 and 31st December 2004. The inclusion diagnostic criteria were the following: (1) Patients with a diagnosis of acute myeloid leukemia, acute lymphoid leukemia, chronic myeloid leukemia, myelodysplastic syndrome (MDS) and myelofibrosis (MMF), who were considered candidates to receive an allogeneic unrelated transplants only after RIC regimens because of their advanced age (55–65 years) or the presence of concurrent medical comorbidities. (2) Patients of any age with the following diagnosis: Hodgkin's disease (HD) relapsed after high-dose chemotherapy or relapsed after 1 year from chemotherapy course and not eligible to high-dose chemotherapy because of mobilization failure. Follicular non-Hodgkin's lymphoma (NHL) relapsed after two courses of standard chemotherapy or high-dose chemotherapy; mantle cell NHL relapsed after one course of standard chemotherapy or high-dose chemotherapy, or lymphoplasmacytic and marginal zone cell NHL relapsed after two courses of standard chemotherapy or high-dose chemotherapy. In addition, patients with B-cell chronic lymphocytic leukemia relapsed after high-dose chemotherapy, mycosis fungoides in advanced phase (>stage III A) or in chemosensitive relapse after two chemotherapy courses and Sezary’s syndrome in chemosensitive relapse after one chemotherapy course. At time of analysis, risk definition for each patient was calculated according to the EBMT score. High-risk patients were defined those with a score ⩾6.7
Donor selection
Donor selection was based on molecular high-resolution typing (4 digits) of the human leukocyte antigen gene loci class I (HLA-A, B and C) and class II (DRB1). In the absence of an 8/8 identical donor, one allele mismatched (class I or II) donor was allowed.
Conditioning regimens
Patients for whom a donor was found, could be prepared for allogeneic transplant using only two preparative regimens: program A, based on the combination of melphalan 30 mg/m2, alemtuzumab (Genzyme Ltd, Haverhill, Suffolk UK) 80 mg, fludarabine 90 mg/m2 and total body irradiation 200 cGy;8 program B, based on thiotepa 10 mg/kg, cyclophosphamide 100 mg/kg and anti-thymocyte globulin (Genzyme Ltd, IDA Industrial Park, Waterford, Ireland) 7.5 mg/kg.9
All patients were treated under local institutional review board guidelines and provided written informed consent for the treatment and for the use of medical information for research.
Statistical methods and definitions
Comparison between proportions was performed using χ2 and Fisher's exact tests. Differences in median times or ages were tested with the Mann–Whitney two sample statistics. Cox models were performed considering UD transplant as time-varying covariate, in order to take into account the bias of patients who were not grafted because of death during the UD search. Multivariable models were performed on the overall population and according to different type of diagnosis, including age, sex and disease risk that was defined high for patients over 60 and for patients who had a previous autologous transplant. Overall survival (OS) was calculated from the UD search activation until deaths from any cause and surviving patients were censored at last follow up, using Kaplan–Meier product limit method. Non-relapse mortality and cumulative incidence of relapse were estimated using competing risks analysis, as far as the cumulative incidence of acute and chronic graft versus host disease (GVHD), considering death without GVHD as competing risks. Cox proportional hazard models with time-varying covariate were established to identify independent prognostic factors to OS. Variables included in the models were sex, age, inclusion diagnostic criteria (1 or 2), source of stem cell (peripheral blood or bone marrow), disease status at transplant (standard phase for patients with at least a 2nd complete remission achieved and high-risk phase for partial remissions, more than 3rd complete remissions, active diseases or relapses), time from donor search activation to transplant (more than 5 months or less), engraftment (yes/no, time-dependent variable), acute and chronic GVHD (yes/no, time-dependent variable).
Results
UD search outcome
The main clinical findings of the 326 patients for whom a donor search was activated are summarized in Table 1: 121 patients (37%) were actually transplanted at a median interval from search activation of 169 days (range: 68–772). Of the 205 patients, who were not transplanted as planned, 192 (59%) stopped the UD search because of death (n=100), ineligibility due to disease progression (n=34), lack or unlikelihood to find a donor (n=11), consent withdrawn (n=8), choice of an alternative program (n=39). This latter group included an autologous transplant (n=1) or allogeneic transplant with a related mismatched (n=15), a cord blood (n=3), or a haploidentical donor (n=2) and other unspecified treatments (n=18). For 13 patients a donor search is still ongoing (Figure 1). The characteristics of patients of this study are summarized in Tables 1 and 2. Patients’ sex and median age were not different between patients who received an allogeneic transplant from an UD and those treated alternatively (P=0.25). HD and NHL as well as acute leukemias were the most common diagnosis. The chosen stem cell source for transplant was peripheral blood in 67 cases (55%) and bone marrow in the other 54 (45%). The majority of patients (80%) were defined at high risk of death according to EBMT criteria.7
Transplants
For patients undergoing transplantation from an UD, neutrophil count recovered to >0.5. × 109/l after a median of 17 days (range, 6–34). Both regimens induced a sustained engraftment in almost 90% of patients. The cumulative incidence of acute GVHD grade II–IV and III–IV, was, respectively, 44% (95% CI, 35–54%) and 20% (95% CI, 13–30%; Figure 2, panel a). The median time to onset of acute GVHD was 40 days after transplantation (range, 12–197). The cumulative incidence of chronic GVHD was 25% (95% CI, 18–34%) with the extensive form occurring in 9% of patients (95% CI, 5–16%; Figure 2, panel b). The median time to onset of chronic GVHD was 115 days after transplantation (range, 90–481). The cumulative incidence of relapse and non-relapse mortality was 33% and 35%, respectively (Figure 3). According to different diagnoses, relapse and non-relapse mortality were, respectively, 42% and 34% for acute leukemias, 32% and 38% for NHL, 34% and 24% for HD, and 16% and 43% for chronic myeloid leukemia, MDS and MMF (data not shown).
UD transplantation versus other treatment
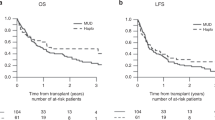
With a median follow up of 2.43 years from the activation of an UD search, the 5-year OS was 39% (95% CI, 30–47%) for patients undergoing an UD transplant and 19% (95% CI, 14–25%) for patients receiving any alternative treatment (Figure 4). To overcome the bias of time to transplant, the therapeutic efficacy of transplant was tested in a multivariable Cox time-dependent model (Table 3). With this approach, we could evaluate the results of multivariate adjusted estimates as to the impact of unrelated transplant on survival in the whole setting and according to the different diagnoses. When considering the whole cohort of 326 patients, an unrelated transplant was not associated with a significantly reduced risk of death (hazard ratio (HR)=0.85, 95% CI, 0.65–1.10). When the analysis was performed separately for different diagnoses, a significant survival advantage with unrelated transplant was shown for patients with acute leukemias (HR=0.60, P=0.049) and NHL (HR=0.47, P=0.008), whereas only a favorable trend was observed for HD patients (HR=0.67, P=0.136; Table 3 and Figure 5). No benefit was evident for patients with chronic myeloid leukemia, MDS, MMF and B-cell chronic lymphocytic leukemia.
By a multivariable model for the prediction of OS (Table 4), a significant decrease of the risk of death was independently associated with a successful engraftment (HR=0.29, 95% CI, 0.13–0.64, P=0.002) and with the incidence of chronic GVHD (HR=0.47, 95% CI, 0.24–0.89, P=0.02). No differences were observed between patients receiving conditioning regimens A or B.
The event free survival at 5 years for the whole patients’ cohort receiving an unrelated transplant was 29% (data not shown). Risk factors predicting event free survival and OS showed the same associations and again, no significant difference was observed between patients treated with either conditioning regimen (data not shown). In acute leukemia and NHL patients the shape of OS and event free survival curves were almost identical, indicating the absence of further significant therapeutic options, in case of disease relapse after allogeneic transplantation. In the case of HD, the event free survival and OS curves diverged, indicating that many patients could benefit from additional therapeutic strategies in case of relapse after allogeneic transplantation (data not shown).
Discussion
The current study was performed to investigate the survival of patients for whom an allogeneic transplantation with an UD was planned and the search of such a donor was formally activated at the Italian Bone Marrow Donor Registry. The main focus of the study was to compare the clinical outcome of the patients who actually received an unrelated allogeneic transplant, with the outcome of patients for whom the transplant was not performed because of the lack of a suitable donor or any other reasons. The time of the UD search activation, according to predefined eligibility criteria, could be considered as a formal declaration of intent to treat. This fact allowed us to compare the outcome of patients undergoing the transplant or not and to analyze the main outcomes of the whole patient population with less selection bias. The choice of a RIC regimen was a priori determined because patients were unfit for conventional transplants because of age, advanced disease or comorbidities, or because they had a diagnosis of HD or NHL or other chronic lymphoid malignancies in a very advanced clinical phase. The planned transplant program was performed in 37% of patients who started the donor search and this can be considered a reasonable result when considering the international registries at the time (2002–2004) this program was carried out. The long-term follow up of this study confirms that an unrelated allogeneic transplant after a RIC regimen may represent a curative option for many patients otherwise ineligible to a conventional allogeneic transplant or with advanced lymphomas. Overall, the 5-year survival of the 121 patients receiving an unrelated transplant (39%) seems to be superior when compared with that of similar patients who were not grafted (19%). However, a simple direct comparison of the two groups of patients is not correct for at least two main obvious selection biases. First, the two groups were been defined prospectively as such when the donor search was activated, and most importantly, the transplant group would include patients surviving long enough for a donor to be available. On the other hand an undue proportion of bad prognosis patients would be assigned to the non-transplant group only because they did not survive long enough to be grafted. In our case, treatment was assigned to the patient by the availability of a suitable donor, which was an external, time-dependent factor, not controlled by the study. Therefore, the use of a time-dependent indicator in multivariable models allowed us to correctly account for the mechanism of treatment allocation. Accordingly, such an appropriate Cox time-dependent analysis was performed and clearly indicates that a true survival benefit could be demonstrated for patients with a diagnosis of acute leukemia and NHL but not for others. Although it is obvious that for the few chronic myeloid leukemia patients enrolled into this study the availability of tyrosine kinase inhibitors has dramatically changed the therapeutic scenario10, 11, 12 for other diseases the interpretation of our results is more complex. Overall, it is likely that, although not curative, effective alternative approaches, may be currently available for patients with an advanced B-cell chronic lymphocytic leukemia13 or HD14 and may not be inferior to an unrelated allogeneic transplant, at least in terms of OS. In addition, although allogeneic transplantation represents a possible definitive curative option for patients with MMF15 and MDS16 it is plausible that, in the absence of an accurate risk oriented patient selection, a survival advantage of the transplant over an appropriate supportive care may be difficult to demonstrate.17 Nonetheless, the lack of a clear cut benefit on survival observed in patients with MMF, MDS or HD may have different explanations. The first obvious possibility relies on the fact that given the relatively low number of these patients and the very advanced phase of their disease even an active and potentially curative therapeutic approach such as the allogeneic transplantation18 could fail to demonstrate an impact on survival. A second possibility may be related to the reduced intensity of the two conditioning regimens, which were designed to minimize transplant-related toxicity. Indeed, in both programs, the treatment intensity was low and the in vivo T-cell depletion, either with alemtuzumab or anti-thymocyte globulin, remarkably high.19 Therefore, it is a distinct possibility that other more intensive conditioning regimens (i.e., those including busulfan or higher doses of melphalan) could have achieved a better impact on survival of patients with MMF,20, 21 MDS,22 HD23 and B-cell chronic lymphocytic leukemia.24 However, when the two regimens were compared, the outcome of the transplant was not affected by the conditioning regimens and GVHD prophylaxis, although this result should be taken with caution, because of the differences in the two patients’ cohorts and the retrospective nature of the study. On the other hand, this analysis does suggests that the success or failure of an UD transplant may be only marginally influenced by the different preparative regimens and controlled clinical trials are needed when new programs are proposed.
In conclusion, finding a donor and proceeding to an UD transplant, offers a survival advantage over not finding a donor, for patients with acute leukemia activating an UD search, and ineligible for a conventional regimen. A similar significant survival advantage was shown for patients with NHL. For chronic leukemias and HD competing, non-transplant therapeutic strategies may possibly offer a similar survival outcome with comparable or even lower toxicity. The role of different conditioning regimens and GVHD prophylaxis with anti-thymocyte globulin or alemtuzumab remains to be elucidated. For this reason, GITMO has conducted and now completed a randomised trial between regimen A and regimen B to evaluate the overall antitumor activity and the safety profile of these two strategies.
References
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010; 363: 2091–2101.
Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood 2005; 105: 1810–1814.
Ballen KK, Shrestha S, Sobocinski KA, Zhang MJ, Bashey A, Bolwell BJ et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant 2010; 16: 358–367.
Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood 2010; 116: 4439–4443.
Devetten MP, Hari PN, Carreras J, Logan BR, van Besien K, Bredeson CN et al. Unrelated donor reduced-intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2009; 15: 109–117.
Karanes C, Nelson GO, Chitphakdithai P, Agura E, Ballen KK, Bolan CD et al. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant 2008; 14: 8–15.
Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer 2009; 115: 4715–4726.
Todisco E, Castagna L, Sarina B, Mazza R, Anastasia A, Balzarotti M et al. Reduced-intensity allogeneic transplantation in patients with refractory or progressive Hodgkin's disease after high-dose chemotherapy and autologous stem cell infusion. Eur J Haematol 2007; 78: 322–329.
Raiola AM, Van Lint MT, Lamparelli T, Gualandi F, Mordini N, Berisso G et al. Reduced intensity thiotepa-cyclophosphamide conditioning for allogeneic haemopoietic stem cell transplants (HSCT) in patients up to 60 years of age. Br J Haematol 2000; 109: 716–721.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009; 27: 6041–6051.
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270.
Robak T, Jamroziak K, Gora-Tybor J, Stella-Holowiecka B, Konopka L, Ceglarek B et al. Comparison of cladribine plus cyclophosphamide with fludarabine plus cyclophosphamide as first-line therapy for chronic lymphocytic leukemia: a phase III randomized study by the Polish Adult Leukemia Group (PALG-CLL3 Study). J Clin Oncol 2010; 28: 1863–1869.
Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL et al. Brentuximabvedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010; 363: 1812–1821.
Kroger N, Holler E, Kobbe G, Bornhauser M, Schwerdtfeger R, Baurmann H et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 2009; 114: 5264–5270.
Alessandrino EP, Della Porta MG, Bacigalupo A, Van Lint MT, Falda M, Onida F et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo ItalianoTrapianto di Midollo Osseo (GITMO). Blood 2008; 112: 895–902.
Cutler C . Allogeneic hematopoietic stem-cell transplantation for myelodysplastic syndrome. Hematol Am Soc Hematol Educ Program 2010; 2010: 325–329.
Corradini P, Sarina B, Farina L . Allogeneic transplantation for Hodgkin's lymphoma. Br J Haematol 2011; 152: 261–272.
Juliusson G, Theorin N, Karlsson K, Frodin U, Malm C . Subcutaneous alemtuzumabvs ATG in adjusted conditioning for allogeneic transplantation: influence of Campath dose on lymphoid recovery, mixed chimerism and survival. Bone Marrow Transplant 2006; 37: 503–510.
Samuelson S, Sandmaier BM, Heslop HE, Popat U, Carrum G, Champlin RE et al. Allogeneic haematopoietic cell transplantation for myelofibrosis in 30 patients 60-78 years of age. Br J Haematol 2010; 153: 76–82.
Robin M, Tabrizi R, Mohty M, Furst S, Michallet M, Bay JO et al. Allogeneic haematopoietic stem cell transplantation for myelofibrosis: a report of the SocieteFrancaise de Greffe de Moelle et de TherapieCellulaire (SFGM-TC). Br J Haematol 2010; 152: 331–339.
Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol 2010; 28: 405–411.
Peggs KS, Hunter A, Chopra R, Parker A, Mahendra P, Milligan D et al. Clinical evidence of a graft-versus-Hodgkin's-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet 2005; 365: 1934–1941.
Dreger P, Dohner H, Ritgen M, Bottcher S, Busch R, Dietrich S et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood 2010; 116: 2438–2447.
Acknowledgements
This work wassupported in part by grants from Associazione Italiana per la Ricerca contro il Cancro (AIRC) and Associazione Italiana Lotta alla Leucemia (AIL). In addition, we wish to thank the following colleagues and their institutions for contributing patients to this study: S Cortelazzo (Bolzano), P Leoni (Ancona),G Milone (Catania), G Marotta (Siena), S Capalbo (Foggia), P Marenco (Milano), F Narni (Modena), M Musso (Palermo), P di Bartolomeo (Pescara), M Petrini (Pisa), G Visani (Pesaro) and I Majolino (Roma). This work is dedicated to the memory of Professor Davide Soligo, who promoted this study and gave a crucial contribution to its study design.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
AR, AB, FC and AB were advisors or paid consultants for Genzyme during study conduct. The remaining authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Rambaldi, A., Bacigalupo, A., Fanin, R. et al. Outcome of patients activating an unrelated donor search: the impact of transplant with reduced intensity conditioning in a large cohort of consecutive high-risk patients. Leukemia 26, 1779–1785 (2012). https://doi.org/10.1038/leu.2012.55
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.55
Keywords
This article is cited by
-
Efficacy of haploidentical hematopoietic stem cell transplantation compared to HLA-matched transplantation for primary refractory acute myeloid leukemia
Annals of Hematology (2018)
-
Long-term follow-up of patients with relapsed or refractory non-Hodgkin’s lymphoma receiving allogeneic stem cell transplantation
Bone Marrow Transplantation (2016)
-
The CIBMTR score predicts survival of AML patients undergoing allogeneic transplantation with active disease after a myeloablative or reduced intensity conditioning: a retrospective analysis of the Gruppo Italiano Trapianto Di Midollo Osseo
Leukemia (2013)