Abstract
Data comparing long-term outcomes in lenalidomide-treated and untreated patients with myelodysplastic syndromes (MDS) with del(5q) are limited. We evaluated clinical outcomes of 295 lenalidomide-treated patients from two clinical trials (MDS-003 and MDS-004) and 125 untreated red blood cell (RBC) transfusion-dependent patients with del(5q) Low- or Intermediate-1 (Int-1)-risk MDS from a large multicenter registry. Risk factors for acute myeloid leukemia (AML) progression and mortality were assessed using Cox proportional hazards models with left truncation to adjust for study entry differences between cohorts. Baseline characteristics were well balanced across cohorts, except for a higher RBC transfusion burden in lenalidomide-treated patients (median, 6 vs 2 units/8 weeks). Median follow-up was 4.3 years from first dose for lenalidomide-treated patients and 4.6 years from diagnosis for untreated patients. Two-year cumulative AML progression incidences were 6.9% (95% confidence interval (CI): 3.3–13.9) and 12.1% (95% CI: 7.0–20.3) and 2-year overall survival (OS) probabilities were 89.9% (95% CI: 84.1–96.0) and 74.4% (95% CI: 66.1–83.7), respectively. AML progression risk was similar in both cohorts (hazard ratio (HR) 0.969, P=0.930); however, lenalidomide treatment was associated with significant improvement in survival (HR 0.597, P=0.012), after adjusting for all other covariates. In conclusion, lenalidomide treatment does not increase AML progression risk, but instead confers a possible survival benefit in RBC transfusion-dependent patients with del(5q) Low- or Int-1-risk MDS.
Similar content being viewed by others
Introduction
In patients with myelodysplastic syndromes (MDS), isolated deletion of the long arm of chromosome 5 (del(5q)) is generally associated with a favorable prognosis.1, 2, 3, 4 However, clinical outcomes in this patient population are adversely affected by red blood cell (RBC) transfusion dependence, additional chromosomal abnormalities, higher bone marrow blast percentage and lower platelet count at baseline.2, 3, 5, 6 In addition, emerging risk factors such as TP53 gene mutations have been associated with worse overall survival (OS) and progression-free survival.7
Lenalidomide (Revlimid; Celgene Corporation, Summit, NJ, USA) has received approval in the USA and several other countries for the treatment of transfusion-dependent anemia in patients with International Prognostic Scoring System (IPSS)-defined Low- or Intermediate-1 (Int-1)-risk MDS and del(5q), with or without additional chromosomal abnormalities. Two large multicenter trials (MDS-003 and MDS-004) investigated the efficacy and safety of lenalidomide in these patients.8, 9 In MDS-003, 67% and 73% of patients treated with lenalidomide (10 mg/day on days 1–21 or 1–28 of each 28-day cycle) achieved RBC transfusion independence for ⩾8 weeks and cytogenetic response, respectively. In MDS-004, treatment with lenalidomide (5 mg/day on days 1–28 and 10 mg/day on days 1–21; both 28-day cycles) resulted in RBC transfusion independence for ⩾8 weeks in 51% and 61% of patients, respectively (P<0.001 for both vs placebo), and cytogenetic response in 25% and 50% of patients, respectively (P<0.001 for both vs placebo). Achievement of RBC transfusion independence for ⩾8 weeks was associated with a significantly reduced risk of progression to acute myeloid leukemia (AML) (hazard ratio (HR) 0.58, P=0.048) and mortality (HR 0.53, P=0.021).9 However, these lenalidomide studies were either single-arm (MDS-003)8 or allowed for crossover from placebo to lenalidomide after 16 weeks (MDS-004),9 thereby precluding long-term assessment of the impact of lenalidomide on AML progression and OS compared with untreated patients.9 Furthermore, data directly comparing the long-term clinical outcomes of RBC transfusion-dependent lenalidomide-treated patients to untreated patients are limited.
In this study we compared the cumulative incidence of AML progression and OS in RBC transfusion-dependent patients with IPSS-defined Low- or Int-1-risk MDS with del(5q) treated with lenalidomide in the MDS-003 and MDS-004 clinical trials, with similar untreated patients from a large multicenter registry. A further objective was to identify potential risk factors for AML progression and mortality in these patients.
Patients and methods
Patients
All lenalidomide-treated patients from two multicenter clinical trials were assessed for inclusion according to protocol eligibility criteria. MDS-003 was a phase 2, single-arm study in RBC transfusion-dependent patients with Low- or Int-1-risk MDS and del(5q) (clinicaltrials.gov identifier NCT00065156). Patients received lenalidomide 10 mg/day either on days 1–21 or days 1–28 of each 28-day cycle.8 MDS-004 was a phase 3, randomized, double-blind, placebo-controlled study conducted in a similar patient population (clinicaltrials.gov identifier NCT00179621). Patients received lenalidomide 10 mg/day on days 1–21 or lenalidomide 5 mg/day on days 1–28, both 28-day cycles, or placebo. Patients randomized to placebo or lenalidomide 5 mg/day who did not achieve a minor erythroid response (⩾50% reduction in RBC transfusion requirements) by week 16 of treatment could cross over to lenalidomide 5 mg/day or 10 mg/day, respectively.9 Transfusion dependence was defined as ⩾2 units/8 weeks in MDS-003 and ⩾1 unit/8 weeks in MDS-004. Additional inclusion and exclusion criteria are described by List et al.8 and Fenaux et al.9
The untreated patient cohort was derived from nine MDS registries in Germany, Austria, Switzerland, France, Czech Republic, Greece, USA and Australia.5 Some of the untreated patients from the French registry were included in a separate report.10 Data were collected using a uniform data set and all except five patients were diagnosed in ‘MDS Foundation Centers of Excellence’, where karyotype, bone marrow blast count, differential count, blood cell counts, RBC transfusion requirements and the exact MDS subtype based on World Health Organization (WHO) criteria were documented and confirmed. In addition, the exact date of diagnosis as determined by bone marrow assessment and cytogenetic analysis was documented.
For the current retrospective analysis, patients eligible for inclusion had to have IPSS-defined Low- or Int-1-risk MDS with del(5q) with or without additional cytogenetic abnormalities, and to be RBC transfusion-dependent, defined according to the WHO-classification-based Prognostic Scoring System as requiring ⩾1 RBC unit/8 weeks at baseline.11 Patients were excluded if they had a platelet count of<50 000/μl or an absolute neutrophil count of<500 cells/μl, reflecting inclusion criteria for the clinical studies. Patients eligible to be included in the lenalidomide cohort had received at least one dose of lenalidomide. The untreated cohort included all eligible registry patients diagnosed after 1982. These patients had received best supportive care only and were allowed to receive RBC transfusions, iron chelation and erythropoiesis-stimulating agents. Twenty-four patients who had received lenalidomide after inclusion in the registry were censored at the time of first dose.5
The MDS-003 and MDS-004 studies were performed according to the Declaration of Helsinki and were approved by institutional review boards or independent ethics committees. All patients gave written informed consent. Data collection and evaluation for the multicenter registry were performed after obtaining institutional review board/ethics committee approval at each individual institution.
Outcome measures and statistical analyses
Primary outcome measures were AML progression and OS in the lenalidomide-treated cohort compared with the untreated cohort. AML was defined according to WHO criteria (⩾20% blasts in the blood or bone marrow).12 All AML cases were confirmed based on local investigator reports and/or death certificate information. Duration of follow-up was measured from the date of first lenalidomide dose received for the lenalidomide cohort and from the date of diagnosis for the untreated cohort. To accurately account for differences in person-time at-risk in these two cohorts due to different starting points for follow-up, left truncation13 was considered appropriate and adopted for all analyses in this study. As lenalidomide-treated patients could not have progressed to AML or died before enrollment in the clinical studies, they are considered at-risk of these end points only after the date of first lenalidomide dose. Without accounting for this phenomenon through left truncation techniques, estimates of AML progression and OS could have been biased. AML progression was determined using a cumulative-incidence estimator in the presence of the competing risk of mortality and left truncation.14, 15 Patients who neither progressed to AML nor died were censored at last observation date. To date, no statistical test has been developed for the comparison of cumulative incidence when using this estimator with both competing risk and left truncation. Therefore, AML progression was also assessed using a left-truncated Kaplan–Meier estimator without competing risk. OS was assessed using a left-truncated Kaplan–Meier estimator. These Kaplan–Meier curves of lenalidomide-treated and untreated patients were compared using a log-rank test, with statistical significance defined as P<0.05.
Risks of AML progression and mortality were evaluated using Cox proportional hazards models with left truncation. In addition to lenalidomide treatment (yes vs no), adjustment was made for major baseline prognostic factors, which were included as continuous variables (age, RBC transfusion burden, hemoglobin level, platelet count and absolute neutrophil count) or as categorical variables (gender, bone marrow blast percentage and cytogenetic complexity (del(5q) plus 1 abnormality vs isolated; and del(5q) plus >1 abnormality vs isolated)). In separate Cox proportional hazards models, lenalidomide treatment, IPSS risk and baseline RBC transfusion burden were considered as potential risk factors for AML progression. Lenalidomide treatment, IPSS risk, baseline age, gender and RBC transfusion burden were considered as potential risk factors for mortality. Potentially significant risk factors (P<0.10) were first identified using univariate Cox proportional hazards models. Final multivariate Cox proportional hazards models were determined using a backward-elimination variable-selection procedure with P>0.10 the significance criterion for elimination. As an exception, lenalidomide treatment was forced to remain in the multivariate models to evaluate the magnitude of risk associated with lenalidomide in the presence of the other covariates.
In addition to assessing the proportional hazards assumption for Cox modeling,16 the robustness of these models was evaluated through several other types of sensitivity analyses. The year of diagnosis was included as an individual covariate in the models. Differences in AML progression and OS were determined for all patients with complete and incomplete covariate information available, and patients who were lost to follow-up were compared with patients not lost to follow-up, with respect to baseline covariates. In addition, models with two different interaction effects were analyzed: the interaction between cohort and transfusion burden; and between cohort and cytogenetic complexity. Lastly, OS was analyzed according to truncation time (<1 vs ⩾1 year) in lenalidomide-treated patients, and in lenalidomide-treated patients with truncation time <1 year vs untreated patients.
The analyses were performed with the SAS software version 9.1.3 (SAS Institute, Cary, NC, USA) and the R software version 2.12.2 (http://www.r-project.org/). All analyses were performed by independent statisticians (ML and JH) from the Institute for Medical Information Sciences, Biometry and Epidemiology of the Ludwig-Maximilians University of Munich, Germany.
Results
Patient disposition and baseline characteristics
A total of 353 patients from the lenalidomide clinical trial cohort and 459 untreated multicenter registry patients were considered for eligibility. A total of 295 lenalidomide-treated and 125 untreated RBC transfusion-dependent patients met inclusion criteria for the present analysis (Figure 1). Patient baseline characteristics and demographics are shown in Table 1. Overall, the lenalidomide-treated and untreated cohorts were well balanced in terms of age, gender, French-American-British classification, IPSS risk, cytogenetic complexity, bone marrow blast percentage, hemoglobin level and platelet and neutrophil counts, with no significant differences between cohorts for these factors. However, RBC transfusion burden was higher in the lenalidomide cohort than in the untreated cohort, with a median of 6 units/8 weeks (range, 1–25) compared with 2 units/8 weeks (range, 1–10), respectively (P<0.001). In addition, there was a lower percentage of patients with WHO-defined isolated del(5q),17 defined as isolated del(5q) and<5% bone marrow blasts, in the lenalidomide-treated cohort than in the untreated cohort (54% vs 68%, respectively, P<0.05). Patients were followed for a median observation time of 4.3 years (range, 0.02–6.8 years) from the first dose received in the lenalidomide-treated cohort and 4.6 years (range, 0.06–19.0 years) from diagnosis in the untreated cohort. Median time from diagnosis to study entry was 2.7 years (range, 0.1–29.2 years) for the lenalidomide cohort.
Patient disposition of the lenalidomide-treated cohort (MDS-003 and MDS-004 trials) and the untreated cohort (multicenter registry). Abbreviations: ANC, absolute neutrophil count; CMML, chronic myelomonocytic leukemia; Int, Intermediate; IPSS, International Prognostic Scoring System; RBC, red blood cell. *Defined as>12 000 leukocytes/μl. †24 patients were censored at the date of first receiving lenalidomide treatment.
AML progression
Overall, 87 patients progressed to AML (68 patients in the lenalidomide-treated cohort and 19 patients in the untreated cohort). The 2- and 5-year cumulative AML incidences were 6.9% and 22.8%, respectively, for lenalidomide-treated patients, and 12.1% and 19.9%, respectively, for untreated patients (Table 2). Median time to AML progression from diagnosis was not reached in either cohort (Figure 2). In a separate Kaplan–Meier comparison that did not consider mortality as a competing risk, there was no significant difference in the probability of AML progression between lenalidomide-treated and untreated patients (log-rank P=0.372).
In the subset of patients with WHO-defined isolated del(5q), the 2- and 5-year cumulative AML incidences were 6.6% and 18.1%, respectively, for lenalidomide-treated patients and 7.4% and 16.9%, respectively, for untreated patients (Table 2). Median time to AML progression was not reached in lenalidomide-treated or untreated patients with WHO-defined isolated del(5q) and there was no significant difference in the probability of AML progression when Kaplan–Meier curves were compared without consideration of competing risk (log-rank P=0.490).
Results of the univariate and multivariate (final) Cox proportional hazards models considering individual baseline covariates as predictors of AML progression in the overall patient cohort are shown in Tables 3 and 4, respectively. The following factors were not associated with an increased risk of AML progression in the final model: lenalidomide-treated vs untreated patients (HR 0.969, P=0.930) and del(5q) plus 1 additional abnormality vs isolated del(5q) (HR 1.095, P=0.786). Baseline factors that were associated with an increased risk of AML progression in the final model were complex cytogenetics (del(5q) plus >1 additional abnormality) vs isolated del(5q) (HR 3.555, P=0.002), bone marrow blast count 5–10% vs <5% (HR 2.158, P=0.019) and a higher RBC transfusion burden (HR 1.090, P=0.041; each 1 unit increase in transfusion burden was associated with a 9% increase in the risk of AML progression). A higher baseline hemoglobin level was associated with a reduced risk of AML progression (HR 0.861, P=0.059).
In models considering IPSS risk, higher RBC transfusion burden was also associated with an increased risk of AML progression (HR 1.098, P=0.027); IPSS Int-1-risk patients were at an increased risk of AML progression compared with Low-risk patients (HR 1.622, P=0.056) (Supplementary Table S1). The result for lenalidomide treatment was consistent with that observed in the multivariate model that considered individual covariates (HR 0.924, P=0.821).
Overall survival
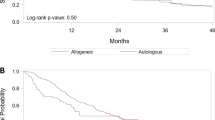
Overall, 220 patients died (161 patients in the lenalidomide-treated cohort and 59 patients in the untreated cohort). The 2- and 5-year OS probabilities were 89.9% and 53.7%, respectively, for the lenalidomide-treated cohort and 74.4% and 40.5%, respectively, for the untreated cohort (Table 2). OS probability for the lenalidomide-treated cohort and the untreated cohort is shown in Figure 3 (log-rank P=0.755). Median OS from diagnosis was 5.2 years (95% confidence interval (CI): 4.5–5.9) for lenalidomide-treated patients and 3.8 years (95% CI: 2.9–4.8) for untreated patients.
In the subset of patients with WHO-defined isolated del(5q), 2- and 5-year OS probabilities were 93.5% and 60.2%, respectively, for lenalidomide-treated patients, and 76.1% and 44.4%, respectively, for untreated patients (Table 2). Median OS was 6.1 years (95% CI: 5.1–6.8) for lenalidomide-treated patients and 4.6 years (95% CI: 3.2–6.1) for untreated patients with WHO-defined isolated del(5q) (log-rank P=0.87 for Kaplan–Meier curve (not shown)).
The results of the univariate and multivariate (final) Cox proportional hazards models considering individual baseline covariates as predictors of OS are shown in Tables 3 and 5, respectively. In the final model with left truncation, lenalidomide treatment was associated with a reduced risk of mortality (HR 0.597, P=0.012) relative to untreated patients. Baseline factors associated with a decreased risk of mortality in the final model were a higher hemoglobin level (HR 0.883, P=0.028), a higher platelet count (HR 0.999, P=0.035) and female gender (HR 0.598, P=0.002). A higher RBC transfusion burden (HR 1.056, P=0.037) and older age (HR 1.049, P<0.001) were associated with an increased risk of mortality.
In a separate Cox proportional hazards model considering IPSS risk, results for lenalidomide treatment, age, gender and RBC transfusion burden were consistent with the findings from the multivariate model that considered individual covariates; however, IPSS risk group had no influence (Supplementary Table S2).
Sensitivity analyses
No evidence for non-proportionality of the Cox models was obtained when evaluated using the Grambsch and Therneau statistic.16 In separate multivariate Cox proportional hazards models considering year of MDS diagnosis as a possible prognostic factor, year of diagnosis was not significantly associated with an increased risk of AML progression (HR 1.042, 95% CI: 0.953–1.141, P=0.369) or mortality (HR 0.992, 95% CI: 0.947–1.040, P=0.750) (Supplementary Table S3). In addition, no differences in AML progression or survival were observed between patients with complete (n=341) and incomplete covariate information available (n=79). There were no differences observed between patients who were lost to follow-up (n=21) and other patients (n=399) with respect to the distribution of baseline covariates. Furthermore, there was no evidence to indicate an interaction with transfusion burden and cohort for mortality or AML progression (Supplementary Table S4). Lenalidomide appeared to have a protective effect against mortality in patients with complex cytogenetics (HR 0.285, 95% CI: 0.075–1.084), although patient numbers with this covariate combination were small (n=17). In the untreated cohort, only three patients with complex cytogenetics were observed, all of whom died without progression to AML; therefore, a robust estimation of the effects concerning complex cytogenetics was not possible. In further sensitivity analyses, lenalidomide-treated patients with a truncation time of <1 year had a higher survival probability compared with those with a truncation time of ⩾1 year (P=0.032) or those in the untreated cohort (P=0.071) (Supplementary Figure S1).
Discussion
This analysis, which compares long-term outcomes of patients from two multicenter trials of lenalidomide with similar patients identified in a large multicenter registry who received best supportive care with or without erythropoietin, provides evidence that treatment with lenalidomide is not associated with an increased risk of AML progression.
Our results are consistent with findings from the MDS-004 study, the only phase 3 randomized, placebo-controlled study of lenalidomide in lower-risk MDS patients with del(5q) conducted to date, which showed that achievement of RBC transfusion independence for ⩾8 weeks with lenalidomide treatment was associated with a significantly reduced risk of AML progression and mortality.9 However, the early crossover design prevented long-term assessment of the impact of lenalidomide treatment on AML progression and OS compared with untreated patients. In addition, despite similar data from MDS-003 indicating that erythroid and cytogenetic responses to lenalidomide are potentially associated with a reduced risk of progression to AML,18 the question of whether lenalidomide treatment confers an increased risk of progression to AML was raised by European regulatory authorities as a result of the seemingly high rate of AML progression observed in the MDS-003 study. However, data from the MDS-003 study should not be compared with patients with MDS and isolated del(5q) defined according to WHO criteria, which are limited to isolated del(5q) and normal bone marrow blast count.17 Inclusion criteria for the clinical trials were IPSS-defined Low- or Int-1-risk MDS, allowing patients with refractory anemia with excess blasts I (RAEB I) as well as those with additional cytogenetic abnormalities to be included. Furthermore, in a mixed cohort of patients with del(5q), only a proportion of patients will be RBC transfusion-dependent, whereas in the study cohorts all patients were transfusion-dependent and many had a high transfusion burden (median 6 units/8 weeks). These prognostic factors are crucial to compare outcome measures.
Patients with del(5q) are commonly believed to be the MDS subgroup with the most favorable prognosis. Nevertheless, progression to AML and OS are highly dependent on additional individual risk factors. In our previous retrospective study of the multicenter registry, outcomes and prognostic variables were evaluated in 381 untreated patients with del(5q).5 It was shown that a high bone marrow blast percentage, complex cytogenetics and platelet count negatively affected the clinical outcomes. Importantly, RBC transfusion dependence was the most powerful covariate for OS and AML progression. These findings confirmed the importance of RBC transfusion dependence as a predictor of clinical outcomes in MDS patients with or without del(5q), as reported in previous studies.5, 11, 19 In the current analysis, which builds on our previous work, RBC transfusion burden emerged as a significant risk factor for AML progression and mortality, although the study was already limited to RBC transfusion-dependent patients.
Adès et al.10 performed a recent retrospective analysis comparing lenalidomide-treated patients with a matched historical cohort of untreated patients with lower-risk MDS and del(5q). A total of 95 lenalidomide-treated patients with a median follow-up duration of 2 years were compared with 99 historical controls; only 35% of patients in the non-lenalidomide-treated cohort were transfusion-independent. This analysis also found that lenalidomide therapy did not increase the risk of AML progression. No significant difference in survival from diagnosis was reported between the lenalidomide-treated cohort and the control cohort.
In the present analysis of lenalidomide-treated vs untreated RBC transfusion-dependent patients with Low- or Int-1-risk MDS and del(5q), lenalidomide treatment was not associated with a greater risk of AML progression as assessed by Cox proportional hazards models with left truncation. In the univariate analysis, no significant impact of lenalidomide on OS was detected. However, in the multivariate model, which adjusts for additional prognostic factors, we found a significant reduction in mortality. These results require confirmation by independent data, ideally in a randomized trial.
This study represents the largest comparative analysis of outcomes in patients treated with and without lenalidomide, and is the first study to focus on outcomes in RBC transfusion-dependent patients. The duration of follow-up was relatively long: up to 6.8 years from the start of therapy in the lenalidomide-treated cohort (median 4.3 years) and up to 19.0 years from diagnosis in the untreated group (median 4.6 years). Both cohorts were well balanced in terms of baseline characteristics, as the inclusion criteria from the two prospective studies were applied to untreated patients from the registry. The only relevant differences between the two cohorts were transfusion burden, which was higher in lenalidomide-treated patients, and the number of patients with WHO-defined isolated del(5q) syndrome, which was higher in untreated patients. Both factors suggest a greater baseline risk for both AML progression and mortality in the lenalidomide-treated patients. Additional risk factors identified in the combined data set were consistent with previous reports, suggesting a representative group of del(5q) patients in both cohorts.2, 3, 5, 6
Limitations of the current study include its retrospective nature and the fact that the lenalidomide-treated cohort was drawn from two separate clinical trials, and compared with a registry cohort of patients established at initial diagnosis. A prospective, randomized, parallel-group trial would be required to confirm these findings. To limit potential bias caused by the different starting points of follow-up in the lenalidomide-treated and untreated cohorts, left truncation was applied for all analyses. This procedure corrects for the different times of entry into the risk set for the two cohorts because all lenalidomide-treated patients experienced an interval between the date of diagnosis and start of treatment (median 2.7 years), whereas registry patients were all followed from the date of diagnosis. The left truncation model adds patients from lenalidomide studies to the respective curve at the point in time when they started lenalidomide therapy. For example, a patient who started lenalidomide therapy 3 years after diagnosis would be added to the curve at the time point of 36 months. During the time before treatment start, the patient has to be removed from the risk set because a patient cannot be at-risk of AML progression or mortality during this interval. However, a patient who was treated with lenalidomide shortly after being diagnosed will be added to the curve immediately and can therefore be compared with a registry patient who was followed from the beginning. Although this is a potential source of bias, its effect can be considered negligible under the reasonable assumption that the patients entering the MDS-003 and MDS-004 clinical studies were representative of untreated patients with MDS with similar survival.20, 21, 22 This method has been applied previously in comparable retrospective analyses including a study comparing erythropoietin-treated and untreated MDS patients by Jädersten et al.23 and the analysis of the effects of lenalidomide treatment by Adès et al.10
Another potential source of bias is the difference in bone marrow assessment schedules in the two cohorts: lenalidomide-treated patients were examined regularly during the clinical trials whereas untreated patients were assessed according to local practice. The number of patients who progressed to AML may, therefore, be underestimated in the untreated cohort, or the date of initial AML diagnosis may have been delayed, which would potentially lower the hazard in untreated patients who were less intensely monitored.
The effects of lenalidomide on the del(5q) clone appear to be dose-dependent based on recent observations that the total dose of lenalidomide received during the first month of therapy is linked to an incremental probability of cytogenetic response.24 Given the different doses and schedules evaluated in the MDS-003 and MDS-004 trials, the inclusion of patients receiving less intense dosing schedules might influence AML progression and OS.
Information on other molecular or genetic abnormalities, such as inactivation of p53, was not available for analysis. The presence of TP53 gene mutations is an independent prognostic factor in lower-risk MDS patients, particularly those with del(5q).7, 25 Inactivation of TP53 represents an important step in the clonal evolution of del(5q) MDS clones, promoting genetic instability and the acquisition of secondary cytogenetic abnormalities, and may also be a marker of lenalidomide resistance.26 Therefore, further study of the impact of TP53 gene mutations in lenalidomide-treated patients is warranted.
Sensitivity analyses provided further support for the findings of the primary analysis. For example, no interaction with transfusion burden and cohort for mortality or AML progression was observed, and lenalidomide appeared to have a protective effect against mortality in patients with complex cytogenetics, although patient numbers with this covariate combination were small (n=17). A further sensitivity analysis compared lenalidomide-treated patients with a brief disease history and thus a short (<1 year) truncation time to untreated patients, which allowed for a closer approximation to a randomized study of newly diagnosed patients. In addition, OS was assessed in lenalidomide-treated patients who started treatment shortly after diagnosis (<1 year) and those who started treatment later (⩾1 year). Results suggest that the survival benefit might be greatest in patients who received lenalidomide early in the course of their disease, potentially due to a smaller number of subclones in such patients, but a randomized study is needed to corroborate this finding.
In conclusion, the results of this large retrospective analysis show that lenalidomide does not increase the risk of AML progression and possibly prolongs survival in RBC transfusion-dependent patients with Low- or Int-1-risk MDS and del(5q).
References
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088.
Giagounidis AA, Germing U, Wainscoat JS, Boultwood J, Aul C . The 5q− syndrome. Hematology 2004; 9: 271–277.
Haase D . Cytogenetic features in myelodysplastic syndromes. Ann Hematol. 2008; 87: 515–526.
Schanz J, Tüchler H, Solé F, Mallo M, Luño E, Cervera J et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol 2012; 30: 820–829.
Germing U, Lauseker M, Hildebrandt B, Symeonidis A, Cermak J, Fenaux P et al. Survival, prognostic factors, and rates of leukemic transformation in 381 untreated patients with MDS and del(5q): a multicenter study. Leukemia 2012; 26: 1286–1292.
Mallo M, Cervera J, Schanz J, Such E, García-Manero G, Luño E et al. Impact of adjunct cytogenetic abnormalities for prognostic stratification in patients with myelodysplastic syndrome and deletion 5q. Leukemia 2011; 25: 110–120.
Kulasekararaj AG, Smith AE, Mian SA, Krishnamurthy P, Mohamedali AM, Lea NC et al. TP53 mutations are restricted predominantly to 5q− syndrome and myelodysplastic syndrome patients with complex cytogenetics, and correlate with adverse prognosis. Blood 2011; 118, (abstract 792).
List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006; 355: 1456–1465.
Fenaux P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood 2011; 118: 3765–3776.
Adès L, Le Bras F, Sebert M, Kelaidi C, Lamy T, Dreyfus F et al. Treatment with lenalidomide does not appear to increase the risk of progression in lower risk myelodysplastic syndromes with 5q deletion. A comparative analysis by the Groupe Francophone des Myelodysplasies. Haematologica 2012; 97: 213–218.
Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007; 25: 3503–3510.
Arber DA, Brunning RD, Orazi RD, Bain BJ . Acute myeloid leukaemia with myelodysplasia-related changes. In: Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al(eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2008, 124–126.
Allison PD . Survival analysis using the SAS system: A practical guide. SAS Institute: Cary, NC, 1995.
Allignol A, Schumacher M, Beyersmann J . Empirical transition matrix of multi-state models: the etm package. J Stat Softw 2011; 38: 1–14.
Meister R, Schaefer C . Statistical methods for estimating the probability of spontaneous abortion in observational studies–Analyzing pregnancies exposed to coumarin derivatives. Reprod Toxicol 2008; 26: 31–35.
Grambsch PM, Therneau TM . Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526.
Hasserjian RP, Le Beau MM, List AF, Bennett JM, Thiele J . Myelodysplastic syndrome with isolated del(5q). In: Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al(eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, France, 2008, 102.
Göhring G, Giagounidis A, Büsche G, Kreipe HH, Zimmermann M, Hellström-Lindberg E et al. Patients with del(5q) MDS who fail to achieve sustained erythroid or cytogenetic remission after treatment with lenalidomide have an increased risk for clonal evolution and AML progression. Ann Hematol 2010; 89: 365–374.
Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol 2005; 23: 7594–7603.
Howards PP, Hertz-Picciotto I, Poole C . Conditions for bias from differential left truncation. Am J Epidemiol 2007; 165: 444–452.
Keiding N, Bayer T, Watt-Boolsen S . Confirmatory analysis of survival data using left truncation of the life times of primary survivors. Stat Med 1987; 6: 939–944.
Tsai W-Y, Jewell NP, Wang M-C . A note on the product-limit estimator under right censoring and left truncation. Biometrika 1987; 74: 883–886.
Jädersten M, Malcovati L, Dybedal I, Della Porta MG, Invernizzi R, Montgomery SM et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol 2008; 26: 3607–3613.
List AF, Giagounidis A, Backstrom JT, Fu T, Fenaux P . Early lenalidomide (LEN) dose intensity and durable RBC-transfusion independence (RBC-TI) in patients (pts) with Low-/Int-1-risk myelodysplastic syndromes (MDS) and del5q. J Clin Oncol 2011; 29 (15 Suppl)(abstract 6522).
Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol 2011; 29: 1971–1979.
Jädersten M, Saft L, Pellagatti A, Göhring G, Wainscoat JS, Boultwood J et al. Clonal heterogeneity in the 5q− syndrome: p53 expressing progenitors prevail during lenalidomide treatment and expand at disease progression. Haematologica 2009; 94: 1762–1766.
Acknowledgements
Celgene Corporation provided funding for this study. The authors received editorial support provided by Nikki Moreland from Excerpta Medica, funded by Celgene Corporation. The authors had full access to the data and are fully responsible for content and editorial decisions for this manuscript.
Author contributions
AK, ML, NAB, JB, AG, JH and UG designed the research; AK, AFL, PF, AAG and UG performed research and collected data; ML and JH performed the statistical analysis. AK and UG wrote the manuscript. All other authors provided significant contribution to the development of the manuscript. All authors had full access to the data, were involved in analyzing and interpreting data, and approved the final version of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
AK has received speaker honoraria from Celgene Corporation. ML declares no conflict of interest. AFL is a consultant for, and has received honoraria and research funding from Celgene Corporation. PF has received honoraria and research funding from Celgene Corporation, Roche and Amgen, and has received honoraria from Johnson & Johnson, Merck, Cephalon and Novartis. AAG is a consultant for, and has received honoraria from Celgene Corporation. NAB, JB and AG are employees of, and hold equity in Celgene Corporation. JH has received research funding from Celgene Corporation. UG has received speaker honoraria and research funding from Celgene Corporation.
Additional information
Presented in abstract form at the 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, USA, December 10–13, 2011.
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kuendgen, A., Lauseker, M., List, A. et al. Lenalidomide does not increase AML progression risk in RBC transfusion-dependent patients with Low- or Intermediate-1-risk MDS with del(5q): a comparative analysis. Leukemia 27, 1072–1079 (2013). https://doi.org/10.1038/leu.2012.369
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.369
Keywords
This article is cited by
-
Current Therapeutic Landscape in Lower Risk Myelodysplastic Syndromes
Current Treatment Options in Oncology (2023)
-
A decade of progress in myelodysplastic syndrome with chromosome 5q deletion
Leukemia (2018)
-
Results of a multicenter prospective phase II trial investigating the safety and efficacy of lenalidomide in patients with myelodysplastic syndromes with isolated del(5q) (LE-MON 5)
Leukemia (2016)
-
8-year-sustained response in a patient with isolated del(5q) myelodysplastic syndrome undergoing continuous treatment with lenalidomide: a case report
memo - Magazine of European Medical Oncology (2016)
-
Lenalidomide for the Treatment of Low- or Intermediate-1-Risk Myelodysplastic Syndromes Associated with Deletion 5q Cytogenetic Abnormality: An Evidence Review of the NICE Submission from Celgene
PharmacoEconomics (2016)