Abstract
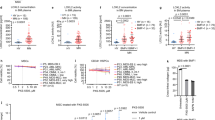
Epigenetic therapies demonstrate significant clinical activity in acute myeloid leukemia (AML) and myelodysplasia (MDS) and constitute an important new class of therapeutic agents. However hematological responses are not durable and disease relapse appears inevitable. Experimentally, leukemic stem/progenitor cells (LSC) propagate disease in animal models of AML and it has been postulated that their relative chemo-resistance contributes to disease relapse. We serially measured LSC numbers in patients with high-risk AML and MDS treated with 5'-azacitidine and sodium valproate (VAL–AZA). Fifteen out of seventy-nine patients achieved a complete remission (CR) or complete remission with incomplete blood count recovery (CRi) with VAL–AZA therapy. There was no significant reduction in the size of the LSC-containing population in non-responders. While the LSC-containing population was substantially reduced in all patients achieving a CR/CRi it was never eradicated and expansion of this population antedated morphological relapse. Similar studies were performed in seven patients with newly diagnosed AML treated with induction chemotherapy. Eradication of the LSC-containing population was observed in three patients all of whom achieved a durable CR in contrast to patients with resistant disease where LSC persistence was observed. LSC quantitation provides a novel biomarker of disease response and relapse in patients with AML treated with epigenetic therapies. New drugs that target this cellular population in vivo are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Burnett A, Wetzler M, Lowenberg B . Therapeutic advances in acute myeloid leukemia. J Clin Oncol 2011; 29: 487–494.
Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S et al. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood 2006; 108: 3271–3279.
Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood 2007; 110: 2302–2308.
Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 2008; 111: 1060–1066.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli G, Giagounidis A et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 2010; 28: 562–569.
Raffoux E, Cras A, Recher C, Boelle PY, de Labarthe A, Turlure P et al. Phase 2 clinical trial of 5-azacitidine, valproic acid, and all-trans retinoic acid in patients with high-risk acute myeloid leukemia or myelodysplastic syndrome. Oncotarget 2010; 1: 34–42.
Bonnet D, Dick JE . Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3: 730–737.
Taussig DC, Miraki-Moud F, Anjos-Afonso F, Perace DJ, Allen K, Ridler C et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood 2008; 112: 568–575.
Taussig DC, Vargaftig J, Mirak-Moud F, Greissinger E, Sharrock K, Luke T et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(-) fraction. Blood 2010; 115: 1976–1984.
Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest 2011; 121: 384–395.
Eppert K, Takenaka K, Lechman ER, Walsron L, Nilsson B, van Galen P et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med 2011; 17: 1086–1093.
Goardon N, Marchi E, Atzberger A, Quek L, Shuh A, Soneji S et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 2011; 19: 138–152.
Ishikawa F, Yoshida S, Saito Y, Hijikata A, Katamura H, Tanaka S et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 2007; 25: 1315–1321.
van Rhenen A, Moshaver B, Kelder A, Feller N, Nieuwint AW, Zweegman S et al. Aberrant marker expression patterns on the CD34+CD38- stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia 2007; 21: 1700–1707.
Ran D, Schubert M, Pietsch L, Taubert I, Wuchter P, Eckstein V et al. Aldehyde dehydrogenase activity among primary leukemia cells is associated with stem cell features and correlates with adverse clinical outcomes. Exp Hematol 2009; 37: 1423–1434.
Gerber JM, Smith BD, Ngwang B, Zhang H, Vala MS, Mosberger L et al. A clinically relevant population of leukemic CD34+CD38- cells in acute myeloid leukemia. Blood 2012; 119: 3571–3577.
Cheson BD, Bennet JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–4649.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 1977; 35: 1–39.
Cox DR . Regression models and life tables. J R Stat Soc. Series B (Methodological) 1972; 34: 187–220.
Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med 2010; 363: 2424–2433.
Walter MJ, Ding L, Shen D, Shao J, Grillot M, McLellan M et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia 2011; 25: 1153–1158.
Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 2009; 361: 1058–1066.
Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 2010; 207: 339–344.
Figueroa ME, Abdel-Waheb O, Lu C, Ward PS, Patel J, Shih A et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010; 18: 553–567.
van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, feller N, Moshaver B et al. The novel AML stem cell associated antigen CLL-1 aids in 483 discrimination between normal and leukemic stem cells. Blood 2007; 110: 2659–2666.
Acknowledgements
Celgene Company provided AZA and an unrestricted educational grant for data management support. We thank patients and clinical teams; Naomi Robertson and Yvonne Morgan for sample processing; Susanna Akiki, Lindsey Bradley and Hayley Newell for CEBPA, NPM1, DNMT3A, IDH1, IDH2 and FISH analysis. CC was supported by CRUK ECMC Funding. NG was supported by an EMBO long-term fellowship and MRC funding. PV acknowledges funding from the Medical Research Council (MRC) Molecular Hematology Unit, MRC Disease Team Award, the Leukemia Lymphoma Research Specialist Program Grant 08030, CRUK Program Grant C7893/A12796 and the National Institute for Health Research (NIHR) Oxford Biomedical Research Center based at Oxford University Hospitals Trust, Oxford. HN was funded by Cure Leukemia (UK registered charity 1100154). NPM1 mutant monitoring studies conducted by AI and DG supported by Leukemia and Lymphoma Research (Grant Ref 07069). SF, RKH, AKB and DG were funded by National Institute for Heath Research Program Grant for assessment of minimal residual disease in the AML17 trial. RM and IL were supported by CIRM Disease Team Grant DR1–01485.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Craddock, C., Quek, L., Goardon, N. et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia 27, 1028–1036 (2013). https://doi.org/10.1038/leu.2012.312
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.312
Keywords
This article is cited by
-
Crosstalk between DNA methylation and hypoxia in acute myeloid leukaemia
Clinical Epigenetics (2023)
-
Therapeutic Targets in Myelodysplastic Neoplasms: Beyond Hypomethylating Agents
Current Hematologic Malignancy Reports (2023)
-
Efficacy of epigenetic agents for older patients with acute myeloid leukemia and myelodysplastic syndrome in randomized controlled trials: a systematic review and network meta-analysis
Clinical and Experimental Medicine (2023)
-
Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: mechanisms of resistance and novel HMA-based therapies
Leukemia (2021)
-
Demethylating therapy increases anti-CD123 CAR T cell cytotoxicity against acute myeloid leukemia
Nature Communications (2021)