Abstract
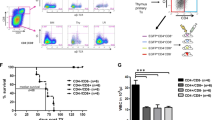
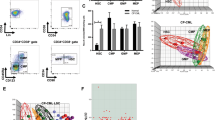
Multiple myeloma is a hematological neoplasm characterized by the accumulation of clonal plasma cells in the bone marrow. Its frequent relapse following achievement of clinical remissions implicates the existence of therapy-resistant myeloma-initiating cells. To date, results on the identity of myeloma-initiating cells have differed. Here, we prospectively identified a myeloma-initiating population by fractionating and transplanting patient bone marrow cells into human bone-bearing immunocompromised mice. Xenotransplantation of fractionated CD138+/CD38high cells from 40% of patients (8/20) led to a repopulation of CD19+CD38low or CD138+CD38+ B-lineage cells in human bone grafts; and these grafts were clonally derived from patient myeloma cells. Meanwhile, CD19+CD38low xenografts were detected in human bone-bearing mice transplanted with CD19+CD38low/− B cells from 8 of 22 samples but were not clonally related to patient myeloma cells. Further fractionation and xenotransplantation of CD138+CD38high cells demonstrated that (CD45low/− or CD19−) CD38high/CD138+ plasma cells, but not (CD45high or CD19+) CD38high/CD138+ plasmablasts enrich for myeloma-initiating cells. Quantitative reverse transcription-PCR of two serially transplantable xenografts, which were CD19−CD138+, revealed that they were Pax5 (a B-cell-specific transactivator)-negative. These results suggest that CD19−CD45low/− fully differentiated plasma cells enrich for long-lived and tumor-initiating cells whereas B cells or plasmablasts do not.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reya T, Morrison SJ, Clarke MF, Weissman IL . Stem cells, cancer, and cancer stem cells. Nature 2001; 414: 105–111.
Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE . Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci USA 2011; 108: 6468–6473.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res 2008; 68: 4311–4320.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63: 5821–5828.
Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009; 27: 1006–1020.
Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V et al. Identification of pancreatic cancer stem cells. Cancer Res 2007; 67: 1030–1037.
Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA 2009; 106: 14016–14021.
Bonnet D, Dick JE . Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3: 730–737.
Miyamoto T, Weissman IL, Akashi K . AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci USA 2000; 97: 7521–7526.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009; 458: 780–783.
Rajkumar SV . Treatment of relapsed or refractory multiple myeloma. In: Kyle RA, Basow DS (eds) UpToDate. UpToDate: Waltham, MA, 2011.
Feo-Zuppardi FJ, Taylor CW, Iwato K, Lopez MH, Grogan TM, Odeleye A et al. Long-term engraftment of fresh human myeloma cells in SCID mice. Blood 1992; 80: 2843–2850.
Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y et al. Characterization of clonogenic multiple myeloma cells. Blood 2004; 103: 2332–2336.
Pilarski LM, Seeberger K, Coupland RW, Eshpeter A, Keats JJ, Taylor BJ et al. Leukemic B cells clonally identical to myeloma plasma cells are myelomagenic in NOD/SCID mice. Exp Hematol 2002; 30: 221–228.
Yaccoby S, Barlogie B, Epstein J . Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood 1998; 92: 2908–2913.
Goldman JP, Blundell MP, Lopes L, Kinnon C, Di Santo JP, Thrasher AJ . Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol 1998; 103: 335–342.
Park CY, Majeti R, Weissman IL . In vivo evaluation of human hematopoiesis through xenotransplantation of purified hematopoietic stem cells from umbilical cord blood. Nat Protoc 2008; 3: 1932–1940.
Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002; 100: 3175–3182.
Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 2007; 104: 973–978.
Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res 2008; 68: 190–197.
Yaccoby S, Epstein J . The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood 1999; 94: 3576–3582.
Yata K, Yaccoby S . The SCID-rab model: a novel in vivo system for primary human myeloma demonstrating growth of CD138-expressing malignant cells. Leukemia 2004; 18: 1891–1897.
Huang SY, Tien HF, Su FH, Hsu SM . Nonirradiated NOD/SCID-human chimeric animal model for primary human multiple myeloma: a potential in vivo culture system. Am J Pathol 2004; 164: 747–756.
McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL . The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 1988; 241: 1632–1639.
Namikawa R, Ueda R, Kyoizumi S . Growth of human myeloid leukemias in the human marrow environment of SCID-hu mice. Blood 1993; 82: 2526–2536.
Epstein J, Yaccoby S . The SCID-hu myeloma model. Methods Mol Med 2005; 113: 183–190.
Pilarski LM, Hipperson G, Seeberger K, Pruski E, Coupland RW, Belch AR . Myeloma progenitors in the blood of patients with aggressive or minimal disease: engraftment and self-renewal of primary human myeloma in the bone marrow of NOD SCID mice. Blood 2000; 95: 1056–1065.
Heppner GH . Tumor heterogeneity. Cancer Res 1984; 44: 2259–2265.
Ichim CV, Wells RA . First among equals: the cancer cell hierarchy. Leuk Lymphoma 2006; 47: 2017–2027.
Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010; 466: 133–137.
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138: 286–299.
Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005; 174: 6477–6489.
Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 2004; 304: 104–107.
Sanz-Rodriguez F, Hidalgo A, Teixido J . Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood 2001; 97: 346–351.
Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA . A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells 2006; 24: 986–991.
Peters M, Jacobs S, Ehlers M, Vollmer P, Mullberg J, Wolf E et al. The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med 1996; 183: 1399–1406.
Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol 2003; 171: 1684–1690.
Kirshner J, Thulien KJ, Martin LD, Debes Marun C, Reiman T, Belch AR et al. A unique three-dimensional model for evaluating the impact of therapy on multiple myeloma. Blood 2008; 112: 2935–2945.
Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007; 21: 1079–1088.
Billadeau D, Ahmann G, Greipp P, Van Ness B . The bone marrow of multiple myeloma patients contains B cell populations at different stages of differentiation that are clonally related to the malignant plasma cell. J Exp Med 1993; 178: 1023–1031.
Chen BJ, Epstein J . Circulating clonal lymphocytes in myeloma constitute a minor subpopulation of B cells. Blood 1996; 87: 1972–1976.
Rasmussen T . The presence of circulating clonal CD19+ cells in multiple myeloma. Leuk Lymphoma 2001; 42: 1359–1366.
Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D . Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci USA 2006; 103: 3304–3309.
Reid S, Yang S, Brown R, Kabani K, Aklilu E, Ho PJ et al. Characterisation and relevance of CD138-negative plasma cells in plasma cell myeloma. Int J Lab Hematol 2010; 32 (6 Pt 1): e190–e196.
Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med 2006; 203: 1859–1865.
Hosen N, Matsuoka Y, Kishida S, Nakata J, Mizutani Y, Hasegawa K et al. CD138-negative clonogenic cells are plasma cells but not B cells in some multiple myeloma patients. Leukemia 2012; e-pub ahead of print 20 March 2012 doi:10.1038/leu.2012.80.
Weissman I . Stem cell research: paths to cancer therapies and regenerative medicine. JAMA 2005; 294: 1359–1366.
Acknowledgements
We thank Dr Stanley Schrier for facilitating the collaboration allowing us to obtain samples of patient bone marrow; Julia Kamp, Rhonda Hewitt and James Puni for obtaining patient consent and clinical specimens; Libuse Jerabek, Theresa Storm and Adriane Mosley for laboratory and mouse management. We especially thank Drs Stephen Willingham, Seth Karten, Ingrid Ibarra and the late Angela Lee for critical comments and the editing of the manuscript. In addition, we thank patients who consented to donate specimens. A part of this research was presented in the 101st AACR meeting in 2010 and in the Lymphoma and Myeloma 2011. Dongkyoon Kim was financially supported by the Irvington Institute Fellowship Program of the Cancer Research Institute (initially by the Irvington Institute for Immunological Research and The Dana Foundation). This research was supported by co-sponsorship (SPO no.: 43710) of the Multiple Myeloma Research Foundation and the Leukemia Lymphoma Society, and by the Ludwig Institute. Irving Weissman is a Daniel K and Virginia Ludwig Professor at Stanford.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Irving L Weissman was a member of the scientific advisory board of Amgen and owns significant Amgen stock; he is a cofounder and director of Stem Cells, Inc. and cofounded Cellerant, Inc. These companies are not in the cancer stem cell field or, if they are now, they were not while Irving L Weissman was an advisor or held stock. The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Kim, D., Park, C., Medeiros, B. et al. CD19−CD45low/−CD38high/CD138+ plasma cells enrich for human tumorigenic myeloma cells. Leukemia 26, 2530–2537 (2012). https://doi.org/10.1038/leu.2012.140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.140
Keywords
This article is cited by
-
Circulating biosignatures in multiple myeloma and their role in multidrug resistance
Molecular Cancer (2023)
-
Mass Cytometry reveals unique phenotypic patterns associated with subclonal diversity and outcomes in multiple myeloma
Blood Cancer Journal (2023)
-
In vitro and ex vivo anti-myeloma effects of nanocomposite As4S4/ZnS/Fe3O4
Scientific Reports (2022)
-
CD34+ myeloma cells with self-renewal activities are therapy-resistant and persist as MRD in cell cycle quiescence
International Journal of Hematology (2022)
-
A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma
Journal of Hematology & Oncology (2021)