Abstract
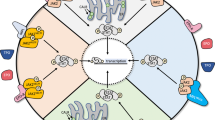
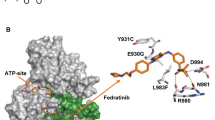
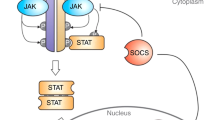
The discovery of JAK2V617F has rejuvenated interest in Janus kinase (JAK)-signal transducer and activator of transcription (STAT), both as an oncogenic pathway and a drug target in BCR-ABL1-negative myeloproliferative neoplasms (MPN). However, the complexity of these diseases in terms of both clonal structure and mutation repertoire makes it unlikely that JAK inhibitor therapy will replicate what has been achieved with imatinib in chronic myeloid leukemia. Consistent with this view, JAK inhibitor therapy in myelofibrosis has not yet produced complete or partial remissions. However, most patients treated with a JAK2 (TG101348) or JAK1/2 (INCB018424) inhibitor experienced substantial improvement in constitutional symptoms and reduction in spleen size; the mechanism of action for INCB018424 includes anti-JAK1-mediated downregulation of proinflammatory cytokines. These observations complicate the choice of primary end points in clinical trials that would be robust enough to support regulatory approval. TG101348 and INCB018424 are the vanguard of JAK inhibitor therapy in myelofibrosis, but newer JAK inhibitors might have a broader spectrum of activity; preliminary results with CYT387 suggest responses in both anemia and splenomegaly. Outstanding issues regarding these drugs include identification of the optimal dosing strategy, their role (if any) in the treatment of polycythemia vera or essential thrombocythemia, and the potential for combining them with other therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Druker BJ . Translation of the Philadelphia chromosome into therapy for CML. Blood 2008; 112: 4808–4817.
Pardanani A . JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia 2008; 22: 23–30.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434: 1144–1148.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005; 7: 387–397.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352: 1779–1790.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365: 1054–1061.
Tefferi A . Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 2010; 24: 1128–1138.
Vannucchi AM, Antonioli E, Guglielmelli P, Pardanani A, Tefferi A . Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: a critical reappraisal. Leukemia 2008; 22: 1299–1307.
Tefferi A, Lasho TL, Huang J, Finke C, Mesa RA, Li CY et al. Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia 2008; 22: 756–761.
Guglielmelli P, Barosi G, Specchia G, Rambaldi A, Lo Coco F, Antonioli E et al. Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood 2009; 114: 1477–1483.
Pardanani A, Lasho TL, Finke C, Hanson CA, Tefferi A . Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007; 21: 1960–1963.
Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007; 356: 459–468.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M et al. MPLW515L Is a Novel Somatic Activating Mutation in Myelofibrosis with Myeloid Metaplasia. PLoS Med 2006; 3: e270.
Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006; 108: 3472–3476.
Vannucchi AM, Antonioli E, Guglielmelli P, Pancrazzi A, Guerini V, Barosi G et al. Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood 2008; 112: 844–847.
Beer PA, Campbell PJ, Scott LM, Bench AJ, Erber WN, Bareford D et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008; 112: 141–149.
Guglielmelli P, Pancrazzi A, Bergamaschi G, Rosti V, Villani L, Antonioli E et al. Anaemia characterises patients with myelofibrosis harbouring Mpl mutation. Br J Haematol 2007; 137: 244–247.
Gery S, Gueller S, Chumakova K, Kawamata N, Liu L, Koeffler HP . Adaptor protein Lnk negatively regulates the mutant MPL, MPLW515L associated with myeloproliferative disorders. Blood 2007; 110: 3360–3364.
Gery S, Cao Q, Gueller S, Xing H, Tefferi A, Koeffler HP . Lnk inhibits myeloproliferative disorder-associated JAK2 mutant, JAK2V617F. J Leukoc Biol 2009; 85: 957–965.
Oh ST, Simonds EF, Jones C, Hale MB, Goltsev Y, Gibbs Jr KD et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood 2010; 116: 988–992.
Pardanani A, Lasho T, Finke C, Oh ST, Gotlib J, Tefferi A . LNK mutation studies in blast-phase myeloproliferative neoplasms, and in chronic-phase disease with TET2, IDH, JAK2 or MPL mutations. Leukemia 2010; 24: 1713–1718.
Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A et al. Mutation in TET2 in myeloid cancers. N Engl J Med 2009; 360: 2289–2301.
Carbuccia N, Murati A, Trouplin V, Brecqueville M, Adelaide J, Rey J et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia 2009; 23: 2183–2186.
Green A, Beer P . Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N Engl J Med 2010; 362: 369–370.
Tefferi A, Lasho TL, Abdel-Wahab O, Guglielmelli P, Patel J, Caramazza D et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia 2010; 24: 1302–1309.
Grand FH, Hidalgo-Curtis CE, Ernst T, Zoi K, Zoi C, McGuire C et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood 2009; 113: 6182–6192.
Jager R, Gisslinger H, Passamonti F, Rumi E, Berg T, Gisslinger B et al. Deletions of the transcription factor Ikaros in myeloproliferative neoplasms. Leukemia 2010; 24: 1290–1298.
Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 2010; 42: 722–726.
Tefferi A . Myelofibrosis with Myeloid Metaplasia. N Engl J Med 2000; 342: 1255–1265.
Gangat N, Caramazza D, Vaidya R, George G, Begna KH, Schwager SM et al. DIPSS-plus: A refined dynamic International Prognostic Scoring System (DIPSS) for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count and transfusion status. J Clin Oncol 2010, in press.
Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A . Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood 2005; 105: 973–977.
Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international internet-based survey of 1179 MPD patients. Cancer 2007; 109: 68–76.
Mesa RA, Schwager S, Radia D, Cheville A, Hussein K, Niblack J et al. The myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res 2009; 33: 1199–1203.
Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for myelofibrosis research and treatment. Blood 2009; 113: 2895–2901.
Siragusa S, Vaidya R, Tefferi A . Hydroxyurea effect on marked splenomegaly associated with Primary Myelofibrosis: response rates and correlation with JAK2V617F Allele Burden. Blood 2009; 114: (abstract 4971).
Martinez-Trillos A, Gaya A, Maffioli M, Arellano-Rodrigo E, Calvo X, Diaz-Beya M et al. Efficacy and tolerability of hydroxyurea in the treatment of the hyperproliferative manifestations of myelofibrosis: results in 40 patients. Ann Hematol 2010, [e-pub ahead of print] doi:10.1007/s00277-010-1019-9.
Mesa RA, Nagorney DS, Schwager S, Allred J, Tefferi A . Palliative goals, patient selection, and perioperative platelet management: outcomes and lessons from 3 decades of splenectomy for myelofibrosis with myeloid metaplasia at the Mayo Clinic. Cancer 2006; 107: 361–370.
Elliott MA, Chen MG, Silverstein MN, Tefferi A . Splenic irradiation for symptomatic splenomegaly associated with myelofibrosis with myeloid metaplasia. Br J Haematol 1998; 103: 505–511.
Tefferi A, Jimenez T, Gray LA, Mesa RA, Chen MG . Radiation therapy for symptomatic hepatomegaly in myelofibrosis with myeloid metaplasia. Eur J Haematol 2001; 66: 37–42.
Riesterer O, Gmur J, Lutolf U . Repeated and preemptive palliative radiotherapy of symptomatic hepatomegaly in a patient with advanced myelofibrosis. Onkologie 2008; 31: 325–327.
Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for myelofibrosis research and treatment (IWG-MRT). Blood 2006; 108: 1497–1503.
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM et al. Longer-term follow up with TG101348 therapy in myelofibrosis confirms sustained improvement in splenomegaly, disease-related symptoms, and JAK2V617F allele burden. Blood 2010, in press (abstract 28895).
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127.
Vannucchi AM . How do JAK2-inhibitors work in myelofibrosis: an alternative hypothesis. Leuk Res 2009; 33: 1581–1583.
Pardanani A, George G, Lasho TL, Hogan WH, Litzow MR, Begna KH et al. A phase I/II study of CYT387, an oral JAK-1/2 inhibitor, in myelofibrosis: significant response rates in anemia, splenomegaly, and constitutional symptoms. Blood 2010, in press (abstract 28767).
Cervantes F, Mesa R, Barosi G . New and old treatment modalities in primary myelofibrosis. Cancer J 2007; 13: 377–383.
Huang J, Tefferi A . Erythropoiesis stimulating agents have limited therapeutic activity in transfusion-dependent patients with primary myelofibrosis regardless of serum erythropoietin level. Eur J Haematol 2009; 83: 154–155.
Mesa RA, Steensma DP, Pardanani A, Li CY, Elliott M, Kaufmann SH et al. A phase 2 trial of combination low-dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasia. Blood 2003; 101: 2534–2541.
Tefferi A, Cortes J, Verstovsek S, Mesa RA, Thomas D, Lasho TL et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood 2006; 108: 1158–1164.
Quintas-Cardama A, Kantarjian HM, Manshouri T, Thomas D, Cortes J, Ravandi F et al. Lenalidomide plus prednisone results in durable clinical, histopathologic, and molecular responses in patients with myelofibrosis. J Clin Oncol 2009; 27: 4760–4766.
Tefferi A, Lasho TL, Mesa RA, Pardanani A, Ketterling RP, Hanson CA . Lenalidomide therapy in del(5)(q31)-associated myelofibrosis: cytogenetic and JAK2V617F molecular remissions. Leukemia 2007; 21: 1827–1828.
Tefferi A, Verstovsek S, Barosi G, Passamonti F, Roboz GJ, Gisslinger H et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol 2009; 27: 4563–4569.
Begna KH, Mesa RA, Pardanani A, Hogan WJ, Litzow MR, McClure RF et al. A phase-2 trial of low dose pomalidomide in myelofibrosis. Leukemia 2010, in press.
Tefferi A, Siragusa S, Hussein K, Schwager SM, Hanson CA, Pardanani A et al. Transfusion-dependency at presentation and its acquisition in the first year of diagnosis are both equally detrimental for survival in primary myelofibrosis--prognostic relevance is independent of IPSS or karyotype. Am J Hematol 2010; 85: 14–17.
Verstovsek S, Passamonti F, Rambaldi A, Barosi G, Rosen P, Levy R et al. A phase 2 study of INCB018424, an oral, selective JAK1/JAK2 inhibitor, in patients with advanced polycythemia vera (PV) and essential thrombocythemia (ET) refractory to hydroxyurea. Blood 2009; 114: 132 (abstract 311).
Lasho TL, Tefferi A, Hood JD, Verstovsek S, Gilliland DG, Pardanani A . TG101348, a JAK2-selective antagonist, inhibits primary hematopoietic cells derived from myeloproliferative disorder patients with JAK2V617F, MPLW515 K or JAK2 exon 12 mutations as well as mutation negative patients. Leukemia 2008; 22: 1790–1792.
Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P et al. A pilot study of the Histone-Deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol 2010; 150: 446–455.
Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010; 115: 3109–3117.
Wernig G, Kharas MG, Okabe R, Moore SA, Leeman DS, Cullen DE et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell 2008; 13: 311–320.
Santos FP, Kantarjian HM, Jain N, Manshouri T, Thomas DA, Garcia-Manero G et al. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood 2010; 115: 1131–1136.
Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A . CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 2009; 23: 1441–1445.
Goh KC, Ong WC, Hu C, Hentze H, Liang AL, Stunkel W et al. SB1317, a potent and orally active FLT3-CDK inhibitor with high anti-tumor efficacy in models of hematological malignancies. Blood 2007; 110: 165a (abstract 538).
Verstovsek S, Odenike O, Scott B, Estrov Z, Cortes J, Thomas DA et al. Phase I dose-escalation trial of SB1518, a novel JAK2/FLT3 inhibitor, in acute and chronic myeloid diseases, including primary or post-essential thrombocythemia/ polycythemia vera myelofibrosis. Blood 2009; 114: 1502 (abstract 3905).
Paquette R, Sokol L, Shah NP, Silver RT, List AF, Clary DO et al. A phase I study of XL019, a selective JAK2 inhibitor, in patients with polycythemia vera. Blood 2008; 112: 971 (abstract 2810).
Shah NP, Olszynski P, Sokol L, Verstovsek S, Hoffman R, List AF et al. A phase I study of XL019, a selective JAK2 inhibitor, in patients with primary myelofibrosis, post-polycythemia vera, or post-essential thrombocythemia myelofibrosis. Blood 2008; 112: 44 (abstract 98).
Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 2009; 16: 487–497.
Acknowledgements
AP's effort for this project was partly supported by the Henry J. Predolin Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AP has received a research grant from Cytopia Inc.
Rights and permissions
About this article
Cite this article
Pardanani, A., Vannucchi, A., Passamonti, F. et al. JAK inhibitor therapy for myelofibrosis: critical assessment of value and limitations. Leukemia 25, 218–225 (2011). https://doi.org/10.1038/leu.2010.269
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.269
Keywords
This article is cited by
-
Interleukin-27 promotes autophagy in human serum-induced primary macrophages via an mTOR- and LC3-independent pathway
Scientific Reports (2021)
-
Polycythemia vera: historical oversights, diagnostic details, and therapeutic views
Leukemia (2021)
-
Early and late stage MPN patients show distinct gene expression profiles in CD34+ cells
Annals of Hematology (2021)
-
Autophagy inhibition potentiates ruxolitinib-induced apoptosis in JAK2V617F cells
Investigational New Drugs (2020)
-
Reversine exhibits antineoplastic activity in JAK2V617F-positive myeloproliferative neoplasms
Scientific Reports (2019)