Abstract
The phosphatidylinositol 3-kinase signal transduction pathway members are often activated in tumor samples from patients with non-Hodgkin's lymphoma (NHL). Everolimus is an oral agent that targets the raptor mammalian target of rapamycin (mTORC1). The goal of this trial was to learn the antitumor activity and toxicity of single-agent everolimus in patients with relapsed/refractory aggressive NHL. Patients received everolimus 10 mg PO daily. Response was assessed after two and six cycles, and then every three cycles until progression. A total of 77 patients with a median age of 70 years were enrolled. Patients had received a median of three previous therapies and 32% had undergone previous transplant. The overall response rate (ORR) was 30% (95% confidence interval: 20–41%), with 20 patients achieving a partial remission and 3 a complete remission unconfirmed. The ORR in diffuse large B cell was 30% (14/47), 32% (6/19) in mantle cell and 38% (3/8) in follicular grade 3. The median duration of response was 5.7 months. Grade 3 or 4 anemia, neutropenia and thrombocytopenia occurred in 14, 18 and 38% of patients, respectively. Everolimus has single-agent activity in relapsed/refractory aggressive NHL and provides proof-of-concept that targeting the mTOR pathway is clinically relevant.
Similar content being viewed by others
Introduction
Patients with non-Hodgkin's lymphoma (NHL) are highly treatable and often curable. However, up to 40% of patients with relapsed diffuse large B-cell NHL (DLBCL) die because of their disease;1 mantle cell lymphoma (MCL) is still not predictably curable,2, 3 and there is substantial need to develop new treatments for transformed lymphoma.4 The PI3K/Akt/mTOR pathway is a key growth and survival pathway for cancer cells.5, 6, 7 The mammalian target of rapamycin (mTOR) kinase, a key member of the pathway, is a 289-kDa serine–threonine kinase that exists in mutually exclusive complexes referred to as mTORC1 and mTORC2.8 Each complex has several components, one of which is mTOR, the catalytic subunit. The key mTORC1 components are regulatory-associated protein of mTOR and mammalian lethal with Sec13 protein 8. mTORC1 positively regulates cell growth and proliferation, and has been characterized as rapamycin sensitive. The components unique to mTORC2 are rapamycin-insensitive companion of mTOR and mammalian stress-activated protein kinase-interacting protein. mTORC2 regulates Akt signaling and is characterized as the rapamycin-insensitive complex.
Rapamycin (sirolimus)9 is the parent drug of the class of mTOR inhibitors that target mTORC1. The rapamycin analogs temsirolimus and everolimus are now approved by the United States Food and Drug Administration for renal cell carcinoma.10, 11 These agents have demonstrated activity against lymphoma cells both in vitro12 and in vivo. For example, single-agent temsirolimus has shown an overall response rate (ORR) of ∼40% with a median duration of response (DR) and time to progression of 6 months in two trials for relapsed MCL.13, 14 A randomized phase III trial in relapsed MCL demonstrated a higher ORR with temsirolimus compared with standard chemotherapy, although the ORR in that trial was only 22%.15 A phase I trial of everolimus in hematological malignancies demonstrated the safety of everolimus at a dose of 10 mg daily.16 We have demonstrated safety and efficacy of single-agent everolimus at the 10 mg daily dose in patients with Waldenstrom's macroglobulinemia,17 small lymphocytic lymphoma18 and relapsed Hodgkin's lymphoma.19
Based on this preclinical work and the excellent toxicity profile of everolimus in previous studies, we performed a phase II clinical trial of single-agent everolimus in patients with relapsed aggressive NHL that had failed standard therapy.
Materials and methods
Laboratory experimental methods
To demonstrate that the everolimus used in this clinical trial indeed did target the mTOR pathway in aggressive NHL, we tested everolimus in vitro using the MCL cell lines Jeko1, Mino and Granta. These were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in RPMI-1640 with 10% fetal bovine serum. The DLBCL cell lines, SUDHL6 and OCILy19, were a generous gift from Dr L Staudt (National Institutes of Health) and were grown in Iscove's Modified Dulbecco's Media (IMDM) with 20% human serum. Everolimus was provided by Novartis Pharmaceuticals (Basel, Switzerland) and was dissolved in 100% dimethyl sulfoxide to a stock concentration of 100 mM and stored at −80 °C. Annexin V-fluorescein isothiocyanate was obtained from BD Biosciences (San Diego, CA, USA). For western blot analysis, 5 × 106 cells were treated with 1, 10 and 50 nM everolimus for 24 h and immunoblotting was performed as previously described.12 Antibodies for phosphorylated mTOR (Ser 2448), S6 ribosomal protein (Ser 235/236) and 4E-BP1 (Thr37/46) were purchased from Cell Signaling Technology (Danvers, MA, USA). For the studies of cell proliferation, the cells were cultured in 96-well round-bottom microtiter plates (Costar, Cambridge, MA, USA) at a density of 5 × 104 cells/well in the presence of various concentration of everolimus. Before harvesting, cells were pulsed with 1 μCi tritiated thymidine (3H-TdR, Amersham, Piscataway, NJ, USA) for 18 h. Incorporation levels of 3H-TdR were determined using a Beckman scintillation counter (GMI, Ramsey, MN, USA).
Cell survival was determined by annexin V/propidium iodide (PI) staining and flow cytometry. In all, 0.5 × 106 cells were cultured with various concentrations of everolimus for 48 h. Cells were then stained using 1 μg/ml annexin V-FITC, washed once in annexin –V-binding buffer and stained with 0.5 μg/ml PI, and analyzed by flow cytometry (FACSCalibur, Becton Dickinson, Franklin Lakes, NJ, USA). Data analysis was performed with FLOW JO software (Becton Dickinson).
Clinical trial patients and methods
A two-stage phase II study was conducted to assess the proportion of patients with relapsed aggressive NHL who achieved a partial response or better after treatment with single-agent everolimus. The study was conducted through the Mayo Clinic Cancer Center and Dana Farber Cancer Center, and was approved by the respective Institutional Review Boards. Patients were eligible if they had previously received therapy and had relapsed or were refractory to their last treatment. There was no limit on the number of previous therapies. Patients were required to have failed or be ineligible for stem cell transplant. The relapse was biopsy proven within 6 months before enrollment. Additional eligibility criteria were age ⩾18 years old, measurable disease by computed tomography or magnetic resonance imaging with at least one lesion >2 cm diameter, life expectancy of ⩾3 months, Eastern Cooperative Oncology Group performance status ⩽2, absolute neutrophil count ⩾1000 × 10(6)/l, platelet count ⩾75 000 × 10(6)/l, hemoglobin ⩾8 g per 100 ml, serum creatinine ⩽2x the upper limit of normal (ULN), serum total bilirubin ⩽2 ULN (or direct bilirubin of <1.5 UNL) and aspartate aminotransferase <3 × ULN (⩽5 × ULN, if liver involvement is present).
Patients received 10 mg of everolimus orally in the fasting state daily; 4 weeks were considered as one cycle. A complete blood count (CBC) was performed weekly during the first cycle and on day 1 of each subsequent cycle. At the time of retreatment, the full dose of everolimus was prescribed, if the platelet count was ⩾40 000 × 10(6)/l, the absolute neutrophil count ⩾1000 × 10(6)/l and there were no grade 3 or 4 non-hematological toxicities (NCI Common Toxicity Criteria version 3.0). Patients who did not meet the retreatment criteria had the dose held until recovery, followed by a stepwise dose modification to 5 mg daily, 5 mg every other day and 5 mg every third day. Patients could receive white blood cell growth factors, if neutropenia developed at physician's discretion. Erythropoietin treatment for anemia was permitted per standard guidelines.
Patients were restaged for response after two and six cycles, and every three cycles thereafter. Responses were categorized using the International Workshop Criteria.20 Patients who progressed or had unacceptable toxicity at any time discontinued therapy. Patients with stable disease after six cycles continued treatment at physician's discretion. Patients who had a complete remission on cycle 6 or later were to receive two additional cycles and then could either discontinue everolimus or continue at physician's discretion. Patients with partial response after six cycles continued until progression or toxicity.
Statistical design
This phase II study used a modified two-stage Simon optimum design21 to assess the efficacy and tolerability of everolimus in patients with aggressive lymphomas, in which the modification is that accrual was not suspended for the stage I analysis. The ORR was estimated by the number of responses divided by the number of evaluable patients. A patient was considered evaluable for response if he received at least one dose of everolimus. A total of 37 evaluable patients were required to test the null hypothesis that the true ORR for this regimen is at most 5% versus the alternative hypothesis that the true ORR is 20% or greater. The study had 90% power, with a 9% type I error rate. A 95% binomial confidence interval for the true ORR was calculated. Because of promising early results, 40 additional patients with aggressive lymphoma were accrued to this study to provide access to everolimus and to better assess the response rate and toxicity profile of this regimen.
DR was defined as the time from the date of documented response to the date of disease progression. Time to progression was defined as the time from registration to the date of disease progression. Patients who had not yet progressed were censored at the date of their last evaluation. Time to discontinuation of active treatment was defined as the time from registration to the date the patient discontinued treatment. Patients who were still receiving treatment at the time of these analyses were censored at the date of their last evaluation. Progression-free survival was defined as the time from registration to the date of disease progression or death from any cause. Overall survival was defined as the time from registration to death from any cause. The distributions of these time-to-event end points were estimated using the Kaplan–Meier method.22 Toxicity was defined as an adverse event classified as being possibly, probably, or definitely related to study treatment.
Results
mTOR pathway is constitutively activated in aggressive lymphoma
Cyclin D1, a cell cycle protein downstream of mTOR, is overexpressed in nearly all cases of MCL. To provide further rationale for using the mTOR inhibitor everolimus in MCL, we evaluated mTOR activity in three mantle cell lines Jeko, Mino and Granta by measuring the phosphorylation profile of mTOR itself and its immediately downstream substrates S6 ribosomal protein (S6RP) and 4E-BP1 by western blot analysis using antibodies directed against mTOR-specific phosphorylation sites for each protein. As shown in Figure 1a, we found that all the three mantle cell lines displayed a high expression of phosphorylated forms of S6RP and 4E-BP1, as well as mTOR on Ser2481. These data suggest that mTOR pathway is constitutively activated in mantle lymphoma and provides a rational target for therapeutic agents such as everolimus.
(a) Immunoblots of proteins from three mantle cell MCL cell lines demonstrating constitutive activation of mTOR and the downstream proteins S6RP and 4EBP1. (b) Everolimus is a potent inhibitor of p-S6RP in both MCL (Jeko) and diffuse large B-cell lymphoma (DHL6) cell lines. Everolimus has less effect on p-4EBP1 in Jeko and no effect on DHL6.
Everolimus attenuates phosphorylation of S6RP and 4E-BP1 in aggressive lymphoma
To confirm that everolimus is actually acting on its intended target in aggressive NHL, we examined the phosphorylation status of the mTOR itself and its target proteins 4E-BP1 and S6RP after treatment with everolimus. Jeko1 (MCL) and DHL6 (DLBCL) cells were cultured with 1, 10 and 50 nM everolimus for 24 h, and western blot analysis was performed using phospho-specific antibodies to mTOR, S6 and 4E-BP1. Treatment with everolimus resulted in the abrogation of S6RP phosphorylation in a dose-dependent manner in both the cell lines (Figure 1b). We also found that everolimus decreased the levels of p-4E-BP1 in a dose-dependent manner in Jeko but not DHL6. We found similar results in previous experiments with rapamycin.12 These results suggest that everolimus is indeed an mTORC inhibitor in aggressive NHL cells.
Blockade of mTOR signaling with everolimus inhibits the growth of aggressive lymphoma, with little effect on apoptosis
Inhibition of mTOR is known to induce cell cycle arrest in tumor cells. Jeko (MCL) and DHL6 (DLBCL) cells were treated with various doses of everolimus for 48 h, followed by an assay of proliferation. Everolimus inhibited the proliferation of both cell lines in a dose-dependent manner with ∼50% reduction in proliferation at 50 nm everolimus compared with controls (Figure 2a). Further experiments were performed to assess the effect of everolimus on lymphoma cell survival. The three MCL lines Jeko, Mino and Granta were treated with various doses of everolimus, and viability was assessed by flow cytometry using annexin/PI staining. Similar to our results with rapamycin,12 we found little effect on survival which was less than 10% inhibition of cell survival in all MCL cell lines, without evidence of any dose-dependent effect (Figure 2b).
Patient characteristics and clinical outcomes
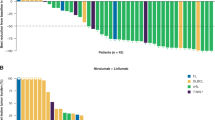
A total of 77 patients were enrolled from September 2005 to September 2008. The patient characteristics and disease types are summarized in Table 1. The ORR was 30% (23/77; 95% confidence interval (CI): 20–41%) with 20 partial response and 3 complete remission unconfirmed (Table 2). A total of 18 additional patients had stable disease and 29 patients progressed by the first assessment. Seven patients went off study before being evaluated for objective status. These results met the study design criteria for success at both the interim and final analysis time points. The ORR for DLBCL patients was 30% (14/47), 32% (6/19) for MCL and 38% (3/8) for follicular lymphoma grade III. None of the three patients with the other disease types (one case of CLL that transformed to DLBCL, one patient with precursor T-lymphoblastic lymphoma and one unspecified high-grade B-cell NHL) responded to everolimus. Tumor responses occurred relatively early, with a median time to response for responders of 2.0 months (range, 1.5–8.7 months). A total of 16 responses occurred after two cycles, 1 response after four cycles, 5 responses after six cycles and 1 response after nine cycles. The ORR in patients with a history of a stem cell transplantation was 32% (8/25).
The median DR for the responders (complete remission unconfirmed/partial response) was 5.7 months (95% CI: 3.6–12.3 months) and five responders remained progression free at 12 months. Overall, 69 patients have had disease progression and 62 patients have died (Figure 3). A total of 50 patients died from disease progression. Other causes of death included an adverse event in four patients (one grade 5 cholecystitis unlikely related to treatment, one grade 5 intra-abdominal hemorrhage unrelated to treatment, one unspecified adverse event while receiving subsequent treatment and one sepsis unrelated to treatment), complications from bone marrow transplant (one patient), graft verses host disease (one patient), unproven disease progression (one patient), death in hospice (one patient) and unknown cause (four patients). The median follow-up on living patients was 24 months (range, 10–48). The median time to progression for all patients was 3.4 months (95% CI: 2.1–4.2), the median progression-free survival was 3.0 months (95% CI: 2.1–3.9) and the median overall survival from study entry was 8.1 months (95% CI: 5.3–12.5).
Safety and tolerability
When considering adverse events of any cause, 43 patients (56%) experienced a grade 3 or higher hematologic adverse event (35% grade 3 and 21% grade 4) and 41 patients (53%) experienced a grade 3 or higher non-hematologic adverse event (40% grade 3, 10% grade 4 and 3% grade 5). Focusing only on adverse events at least possibly attributable to the study therapy (Table 3), 42 patients (55%) experienced a grade 3 or higher hematologic toxicity (35% grade 3 and 19% grade 4). Grade 3 or 4 anemia, neutropenia and thrombocytopenia occurred in 14, 18 and 38% of the patients, respectively. A total of 22 patients (29%) experienced a grade 3 or higher non-hematologic toxicity (23% grade 3 and 5% grade 4). The most frequent non-hematologic grade 3 or 4 toxicities were hyperglycemia (8%), fatigue (8%) and mucositis (3%).
Overall, 52 patients (68%) had at least one grade 3/4 toxicity at least possibly related to everolimus. Although most patients experienced toxicity, as described in Table 3, the incidence of most of these complications was low and they were manageable with dose interruptions and dose reductions. Thrombocytopenia was the cause of most dose reductions and was rapidly reversible with drug delays of typically 1 week. Three patients experienced grade 3 respiratory tract infection. Two additional patients had grade 3 pulmonary toxicity—one with cough and the other with dyspnea, hypoxia and non-infectious pneumonitis.
A total of 77 patients received a median of 2.0 months of everolimus treatment (range, 1 day–45.3+ months). A total of 75 patients have discontinued treatment, with the most common reasons being disease progression (51 patients), adverse events (6 patients) and refusal (4 patients). The median time to discontinuation of treatment was 2.0 months (95% CI: 1.9–2.9 months). Five patients remained on drug for at least 12 months, four patients for ⩾18 months and four patients for ⩾24 months.
Of the 77 patients who received at least one dose of everolimus, 7 did not complete cycle 1 and 7 additional patients went off at the end of cycle 1, leaving 63 pts who received >1 cycle. The median number of cycles at 10 mg daily was two (range, 1–12), with 87% (55/63) of patients receiving 10 mg daily at cycle 2. Of the 55 patients who received more than one cycle at the full dose level, 14 eventually required a dose reduction. A total of 28 patients (36%) had dose reductions (19 patients) or treatment delays (17 patients). The most common causes of dose reductions were hematologic toxicity (thrombocytopenia or neutropenia), stomatitis, edema and rash. Treatment delays were due to hematologic toxicity (thrombocytopenia and neutropenia) and infection.
Discussion
The PI3K pathway has been demonstrated to be constitutively activated in the majority of B-cell lymphomas as manifested by phosphorylation of S6K and 4E-BP1 (Figure 1).12, 23, 24 Preclinical studies in cell lines and primary samples of aggressive NHL have demonstrated that rapamycin or its analogs has a significant antiproliferative effect on these specimens but does not substantially inhibit tumor cell survival.12 In this study, we again demonstrated that everolimus, the mTOR inhibitor used in this clinical study, efficiently inhibits mTOR activity in aggressive lymphoma in-vitro as shown by decreased cell proliferation and inhibits levels of phosphorylation of the downstream targets S6RP and 4E-BP1. Other investigators have found similar results.25 Everolimus can also sensitize MCL and DLBCL cell lines to a variety of cytotoxic agents, including doxorubicin and bortezomib.26 This preclinical data provided the rationale to perform this large phase II study of single-agent everolimus.
Although the ORR result of 30% achieved with everolimus in this trial is modest, it is indeed significant for several reasons. Firstly, the relapsed aggressive patient population is particularly difficult to treat because they are typically older adults, heavily pretreated and have tumors with high kinetic activity. In this study, the median age of the patients was 70 years and they had received a median of three previous therapies. The aggressive nature of the tumors in these patients is reflected in the substantial number of patients (18%) that went off-study after only one cycle. Thus, finding an ORR of 30% in this group is meaningful and provides the rationale for further studies of combinations. Secondly, the everolimus treatment in this study was single agent providing clear proof-of-principle that the responses were due to everolimus and that this class of agents may play an important role in the treatment of NHL. The fact that the ORR was 30% with a median DR for responders of nearly 6 months fits with the preclinical data in NHL that suggested that inhibition of mTOR would produce responses. Thirdly, oral everolimus therapy was convenient and tolerable even for extended periods of time.
The everolimus results for relapsed DLBCL are similar to the ORR and DR found with single-agent lenalidomide.27 The 32% ORR with everolimus in relapsed MCL is similar to the 40% that we found in our previous trials of temsirolimus13, 14 and higher than the 22% determined in the phase III trial.15 In the first trial of lenalidomide for relapsed NHL, Wiernik et al.27 found tumor responses in 8 of 15 (53%) patients with MCL. These pilot results, if confirmed, suggest that both mTOR inhibitors and immunodulatory agents are active in MCL.
Everolimus and other mTOR inhibitors are generally well tolerated. The mTOR inhibitors as a class can produce apthous-type mouth ulcers, hyperlipidemia, dysgeusia that can produce mild weight loss, thrombocytopenia and fatigue. Most all of these toxicities can be managed with dose interruption and dose reduction after the toxicity resolves. In the case of hyperlipidemia, the addition of a statin drug is often required to maintain mTOR inhibitor dose intensity, especially if the patient has an antitumor response and will be on the drug for an extended period of time. We observed an 1% incidence of grade 3 dyspnea, a rate similar to that found in other studies with everolimus28 or temsirolimus.10, 15 The most recent report of everolimus used in relapsed neuroendocrine tumors had a 13% incidence of pulmonary toxicity.29
The promising activity of mTOR inhibition also has implications for adjuvant therapy in lymphoma. Armand et al.30 found that patients with NHL or Hodgkin's lymphoma who underwent graft verses host disease prophylaxis with sirolimus after reduced intensity allogeneic stem cell transplantation had an improved survival relative to patients who received prophylaxis with a calcineurin inhibitor plus methotrexate. Trials are underway in DLBCL to test whether adjuvant everolimus after standard rituximab-based chemoimmunotherapy has induced a complete remission can lower the risk of relapse.
The lack of response of many patients with relapsed aggressive NHL to everolimus in this trial indicates that a mechanism or mechanisms of resistance to mTORC1 inhibition exists or develops in these tumor cells. We have demonstrated that when NHL cells are treated with prototypical mTORC1 inhibitors such as rapamycin, although mTORC1 is blocked, the cells activate Akt through mTORC2 signaling, thus providing an escape mechanism.12 This has led to a search for agents that will overcome this resistance or be synergistic with mTORC1 inhibitors. Sorafenib, a novel multikinase inhibitor that acts through inhibition of Raf kinase and VEGF receptors, has synergistic activity in vitro with rapamycin.31 This provided the rationale for a phase I/II ongoing trial of sorafenib and everolimus for relapsed B-cell malignancies. Rapamycin also has demonstrated synergy with the histone deacetylase inhibitor LBH589, and a phase I trial of LBH589 and everolimus are ongoing at multiple centers.12 Understanding the mechanisms of resistance in lymphoma cells when exposed to these targeted agents will shed further light on the pathways important to the growth and maintenance of malignant B cells and provide important leads into new mTOR-based combinations for aggressive NHL.
References
Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 2006; 24: 3121–3127.
Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 2008; 112: 2687–2693.
Inwards DJ, Fishkin PA, Hillman DW, Brown DW, Ansell SM, Kurtin PJ et al. Long-term results of the treatment of patients with mantle cell lymphoma with cladribine (2-CDA) alone (95-80-53) or 2-CDA and rituximab (N0189) in the North Central Cancer Treatment Group. Cancer 2008; 113: 108–116.
Al-Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma. J Clin Oncol 2008; 26: 5165–5169.
Witzig TE, Kaufmann SH . Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Curr Treat Options Oncol 2006; 7: 285–294.
Cully M, You H, Levine AJ, Mak TW . Beyond PTEN mutations: the PI3 K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 2006; 6: 184–192.
LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA . Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat 2008; 11: 32–50.
Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N . Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta 2010; 1804: 433–439.
Sehgal SN, Baker H, Vezina C . Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975; 28: 727–732.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356: 2271–2281.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008; 372: 449–456.
Gupta M, Ansell SM, Novak AJ, Kumar S, Kaufmann SH, Witzig TE . Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood 2009; 114: 2926–2935.
Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 2005; 23: 5347–5356.
Ansell SM, Inwards DJ, Rowland Jr KM, Flynn PJ, Morton RF, Moore Jr DF et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer 2008; 113: 508–514.
Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 2009; 27: 3822–3829.
Yee KW, Zeng Z, Konopleva M, Verstovsek S, Ravandi F, Ferrajoli A et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res 2006; 12: 5165–5173.
Ghobrial IM, Gertz M, Laplant B, Camoriano J, Hayman S, Lacy M et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenstrom macroglobulinemia. J Clin Oncol 2010; 28: 1408–1414.
Zent CS, LaPlant BR, Johnston PB, Call TG, Habermann TM, Micallef IN et al. The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer 2010; 116: 2201–2207.
Johnston PB, Inwards DJ, Colgan JP, Laplant BR, Kabat BF, Habermann TM et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol 2010; 85: 320–324.
Cheson B, Horning S, Coiffier B, Shipp M, Fisher R, Connors J et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphoma. J Clin Oncol 1999; 17: 1244–1253.
Simon R . Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10.
Kaplan E, Meier P . Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Wlodarski P, Kasprzycka M, Liu X, Marzec M, Robertson ES, Slupianek A et al. Activation of mammalian target of rapamycin in transformed B lymphocytes is nutrient dependent but independent of Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, insulin growth factor-I, and serum. Cancer Res 2005; 65: 7800–7808.
Dutton A, Reynolds GM, Dawson CW, Young LS, Murray PG . Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol 2005; 205: 498–506.
Haritunians T, Mori A, O’Kelly J, Luong QT, Giles FJ, Koeffler HP . Antiproliferative activity of RAD001 (everolimus) as a single agent and combined with other agents in mantle cell lymphoma. Leukemia 2007; 21: 333–339.
Wanner K, Hipp S, Oelsner M, Ringshausen I, Bogner C, Peschel C et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol 2006; 134: 475–484.
Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol 2008; 26: 4952–4957.
Ellard SL, Clemons M, Gelmon KA, Norris B, Kennecke H, Chia S et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND163. J Clin Oncol 2009; 27: 4536–4541.
Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol 2010; 28: 69–76.
Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol 2008; 26: 5767–5774.
Ramakrishnan V, Timm M, Haug JL, Kimlinger TK, Wellik LE, Witzig TE et al. Sorafenib, a dual Raf kinase/vascular endothelial growth factor receptor inhibitor has significant anti-myeloma activity and synergizes with common anti-myeloma drugs. Oncogene 2010; 29: 1190–1202.
Acknowledgements
Research supported in part by the NIH grants CA127433, CA112904 and CA97274, and the Predolin Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
No author received personal compensation directly from Novartis to conduct the trial. Rather, Mayo Clinic and Dana Farber received research support for the conduct of the clinical trial from Novartis. In 2007 TEW participated in a advisory board (personal uncompensated; Mayo received the compensation) with Novartis.
Additional information
Presented in part at the European Hematology Association Annual Meeting, Berlin, Germany, 2009.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Witzig, T., Reeder, C., LaPlant, B. et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia 25, 341–347 (2011). https://doi.org/10.1038/leu.2010.226
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.226
Keywords
This article is cited by
-
Evolving treatment patterns and improved outcomes in relapsed/refractory mantle cell lymphoma: a prospective cohort study
Blood Cancer Journal (2023)
-
mTOR inhibition amplifies the anti-lymphoma effect of PI3Kβ/δ blockage in diffuse large B-cell lymphoma
Leukemia (2023)
-
Update in Diagnosis and Management of Primary Cutaneous B-Cell Lymphomas
American Journal of Clinical Dermatology (2022)
-
New agents and regimens for diffuse large B cell lymphoma
Journal of Hematology & Oncology (2020)
-
Genetic alterations and their clinical implications in DLBCL
Nature Reviews Clinical Oncology (2019)