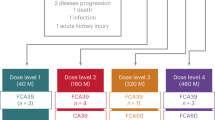
Abstract
Ligation of CD40 on chronic lymphocytic leukemia (CLL) cells induces phenotypic and biochemical changes that facilitate CLL cell–T cell interactions and enhances the sensitivity of CLL cells to clearance by adaptive and innate immune-effector mechanisms. CLL cells can be transduced to express CD40 ligand (CD154) using a replication-defective adenovirus vector, thereby cross-linking CD40 on transduced and non-transduced, bystander CLL cells. In a previous study, patients received infusions of autologous CLL cells, transduced to express murine CD154 (mCD154), which induced anti-leukemic immune responses, but also anti-mCD154 antibodies. In this study, we report a phase I study, in which patients were infused with 1 × 108, 3 × 108 or 1 × 109 autologous CLL cells transduced ex vivo to express ISF35, a humanized, membrane-stable CD154. Infusions were well tolerated and consistently followed by reductions in blood lymphocyte counts and lymphadenopathy. After infusion, circulating CLL cells had enhanced or de novo expression of CD95, DR5, p73 and Bid, which enhanced their susceptibility to death-receptor-mediated or drug-induced apoptosis, including CLL cells with deletions at 17p13.1 (del(17p)). Two patients who had CLL with del(17p) had subsequent chemoimmunotherapy and responded well to treatment. In summary, infusions of autologous, ISF35-transduced CLL cells were well tolerated, had biological and clinical activity, and might enhance the susceptibility of CLL cells with del(17p) to chemoimmunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wierda WG, Keating MJ, O′Brien S . Chronic lymphocytic leukemia. In: DeVita VT, Lawrence TS, Rosenberg SA (eds). Cancer: Principles & Practice of Oncology, 8th edn. Lippincott Williams & Wilkins: Philadelphia, PA, 2008, pp 2278–2291.
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005; 23: 4079–4088.
Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008; 112: 975–980.
Hallek M, Fingerle-Rowson G, Fink A-M, Busch R, Mayer J, Hensel M et al. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression-free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL). Blood 2008; 112, (abstract no. 325).
Hallek M, Fingerle-Rowson G, Fink A-M, Busch R, Mayer J, Hensel M et al. First-line treatment with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) improves overall survival (OS) in previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL): results of a randomized phase III trial on behalf of an international group of investigators and the German CLL Study Group. Blood 2009; 114, (abstract no. 535).
Robak T, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol 2010; 28: 1756–1765.
Ranheim EA, Kipps TJ . Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med 1993; 177: 925–935.
Kato K, Cantwell MJ, Sharma S, Kipps TJ . Gene transfer of CD40-ligand induces autologous immune recognition of chronic lymphocytic leukemia B cells. J Clin Invest 1998; 101: 1133–1141.
Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ . CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood 2000; 96: 2917–2924.
Fukuda T, Chen L, Endo T, Tang L, Lu D, Castro JE et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci USA 2008; 105: 3047–3052.
Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996; 87: 4990–4997.
Chu P, Deforce D, Pedersen IM, Kim Y, Kitada S, Reed JC et al. Latent sensitivity to Fas-mediated apoptosis after CD40 ligation may explain activity of CD154 gene therapy in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2002; 99: 3854–3859.
Chu P, Wierda WG, Kipps TJ . CD40 activation does not protect chronic lymphocytic leukemia B cells from apoptosis induced by cytotoxic T lymphocytes. Blood 2000; 95: 3853–3858.
Dicker F, Kater AP, Fukuda T, Kipps TJ . Fas-ligand (CD178) and TRAIL synergistically induce apoptosis of CD40-activated chronic lymphocytic leukemia B cells. Blood 2005; 105: 3193–3198.
Dicker F, Kater AP, Prada CE, Fukuda T, Castro JE, Sun G et al. CD154 induces p73 to overcome the resistance to apoptosis of chronic lymphocytic leukemia cells lacking functional p53. Blood 2006; 108: 3450–3457.
Ozaki T, Nakagawara A . p73, a sophisticated p53 family member in the cancer world. Cancer Sci 2005; 96: 729–737.
Pluta A, Nyman U, Joseph B, Robak T, Zhivotovsky B, Smolewski P . The role of p73 in hematological malignancies. Leukemia 2006; 20: 757–766.
Muller M, Schilling T, Sayan AE, Kairat A, Lorenz K, Schulze-Bergkamen H et al. TAp73/Delta Np73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ 2005; 12: 1564–1577.
Foster AE, Okur FV, Biagi E, Lu A, Dotti G, Yvon E et al. Selective elimination of a chemoresistant side population of B-CLL cells by cytotoxic T lymphocytes in subjects receiving an autologous hCD40L/IL-2 tumor vaccine. Leukemia 2010; 24: 563–572.
Acknowledgements
This clinical trial and correlative laboratory studies were supported by the research funding provided by Memgen, LLC. This project was a collaboration formed under the CLL Research Consortium.
Author contributions
WG Wierda designed and performed the research, collected, analyzed and interpreted the data, performed statistical analysis and wrote the article. JE Castro, R Aguillon and D Sampath performed the research and contributed analytical tools. A Jalayer collected the data. J McMannis contributed analytical tools. CE Prussak designed the research. M Keating performed the research. TJ Kipps designed the research and wrote the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
WG Wierda, JE Castro, D Sampath and J McMannis received research grant funding to complete the work included herein. R Aguillon, A Jalayer and M Keating declare no conflict of interest. CE Prussak has a financial interest in MemGen, the sponsor of this trial. TJ Kipps has been a scientific advisor to Memgen and inventor of technology patented by the University of California and licensed to Memgen.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Wierda, W., Castro, J., Aguillon, R. et al. A phase I study of immune gene therapy for patients with CLL using a membrane-stable, humanized CD154. Leukemia 24, 1893–1900 (2010). https://doi.org/10.1038/leu.2010.191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.191
Keywords
This article is cited by
-
Programming CAR T cells to enhance anti-tumor efficacy through remodeling of the immune system
Frontiers of Medicine (2020)
-
Haematological malignancies: at the forefront of immunotherapeutic innovation
Nature Reviews Cancer (2015)