Abstract
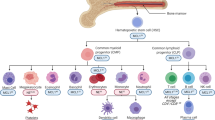
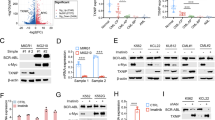
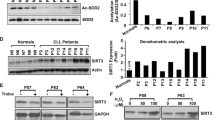
Bcr-Abl causes chronic myelogenous leukemia, a myeloproliferative disorder characterized by clonal expansion of hematopoietic progenitor cells. In this study, inducible expression of Bcr-Abl in TonB.210 cells is associated with increased production of intracellular reactive oxygen species (ROS), which is thought to play a role in survival signaling when generated at specific levels. Elevated ROS in Bcr-Abl-expressing cells were found to activate PI3k/Akt pathway members such as Akt and GSK3β as well as downstream targets β-catenin and Mcl-1. The activation of these proteins was inhibited by the flavoprotein inhibitor diphenyleneiodonium, which is commonly used to inhibit NADPH oxidase (Nox). This indicated that increased ROS might be related to increased activity of one member of the Nox family. Knock-down experiments using siRNA suggest that Nox-4 is the main source of increased ROS following Bcr-Abl expression. We showed that Bcr-Abl-induced ROS could also increase survival pathway signaling through redox inhibition of PP1α, a serine threonine phosphatase that negatively regulates the PI3k/Akt pathway. Overall our results demonstrate that Bcr-Abl expression increases Nox-4-generated ROS, which in turn increases survival signaling through PI3k/Akt pathway by inhibition of PP1α, thus contributing to the high level of resistance to apoptosis seen in these Bcr-Abl-expressing cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rowley JD . Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973; 243: 290–293.
Shtivelman E, Lifshitz B, Gale RP, Canaani E . Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature 1985; 315: 550–554.
Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR . Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell 2006; 21: 749–760.
Millward TA, Zolnierowicz S, Hemmings BA . Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci 1999; 24: 186–191.
O'Loghlen A, Perez-Morgado MI, Salinas M, Martin ME . Reversible inhibition of the protein phosphatase 1 by hydrogen peroxide. Potential regulation of eIF2 alpha phosphorylation in differentiated PC12 cells. Arch Biochem Biophys 2003; 417: 194–202.
Rao RK, Clayton LW . Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem Biophys Res Commun 2002; 293: 610–616.
Bedard K, Krause KH . The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87: 245–313.
Lambeth JD . NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4: 181–189.
Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T et al. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem 2000; 275: 24273–24278.
Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D et al. Activation of the PI3K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood 2005; 105: 1717–1723.
Klucher KM, Lopez DV, Daley GQ . Secondary mutation maintains the transformed state in BaF3 cells with inducible BCR/ABL expression. Blood 1998; 91: 3927–3934.
Krishna CM, Liebmann JE, Kaufman D, DeGraff W, Hahn SM, McMurry T et al. The catecholic metal sequestering agent 1,2-dihydroxybenzene-3,5-disulfonate confers protection against oxidative cell damage. Arch Biochem Biophys 1992; 294: 98–106.
Casas J, Gorchs G, Sanchez-Baeza F, Teixidor P, Messeguer A . Inhibition of rat liver microsomal lipid peroxidation elicited by simple 2,2-dimethylchromenes and chromans structurally related to precocenes. J Agric Food Chem 1992; 40: 585–590.
England K, O’Driscoll C, Cotter TG . Carbonylation of glycolytic proteins is a key response to drug-induced oxidative stress and apoptosis. Cell Death Differ 2004; 11: 252–260.
Mackey AM, Sanvicens N, Groeger G, Doonan F, Wallace D, Cotter TG . Redox survival signalling in retina-derived 661W cells. Cell Death Differ 2008; 15: 1291–1303.
Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B et al. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem 1997; 272: 843–851.
Fleury C, Mignotte B, Vayssiere JL . Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002; 84: 131–141.
Clevers H . Wnt/beta-catenin signaling in development and disease. Cell 2006; 127: 469–480.
Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med 2004; 351: 657–667.
Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H, Florian S, Sonneck K et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood 2005; 105: 3303–3311.
Ueyama T, Geiszt M, Leto TL . Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol 2006; 26: 2160–2174.
Chen J, Wall NR, Kocher K, Duclos N, Fabbro D, Neuberg D et al. Stable expression of small interfering RNA sensitizes TEL-PDGFbetaR to inhibition with imatinib or rapamycin. J Clin Invest 2004; 113: 1784–1791.
Resjo S, Oknianska A, Zolnierowicz S, Manganiello V, Degerman E . Phosphorylation and activation of phosphodiesterase type 3B (PDE3B) in adipocytes in response to serine/threonine phosphatase inhibitors: deactivation of PDE3B in vitro by protein phosphatase type 2A. Biochem J 1999; 341 (Part 3): 839–845.
Stone JR, Yang S . Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 2006; 8: 243–270.
Hickey FB, Cotter TG . BCR-ABL regulates phosphatidylinositol 3-kinase-p110gamma transcription and activation and is required for proliferation and drug resistance. J Biol Chem 2006; 281: 2441–2450.
Ress A, Moelling K . Bcr is a negative regulator of the Wnt signalling pathway. EMBO Rep 2005; 6: 1095–1100.
Miyano K, Sumimoto H . Role of the small GTPase Rac in p22phox-dependent NADPH oxidases. Biochimie 2007; 89: 1133–1144.
Miyano K, Ueno N, Takeya R, Sumimoto H . Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem 2006; 281: 21857–21868.
Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG . Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 2006; 18: 69–82.
Etoh T, Inoguchi T, Kakimoto M, Sonoda N, Kobayashi K, Kuroda J et al. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 2003; 46: 1428–1437.
Eng C . PTEN: one gene, many syndromes. Hum Mutat 2003; 22: 183–198.
LaMontagne Jr KR, Flint AJ, Franza Jr BR, Pandergast AM, Tonks NK . Protein tyrosine phosphatase 1B antagonizes signalling by oncoprotein tyrosine kinase p210 bcr-abl in vivo. Mol Cell Biol 1998; 18: 2965–2975.
Tauchi T, Feng GS, Shen R, Song HY, Donner D, Pawson T et al. SH2-containing phosphotyrosine phosphatase Syp is a target of p210bcr-abl tyrosine kinase. J Biol Chem 1994; 269: 15381–15387.
Chen CS, Weng SC, Tseng PH, Lin HP, Chen CS . Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. J Biol Chem 2005; 280: 38879–38887.
Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP . Identification of a tumour suppressor network opposing nuclear Akt function. Nature 2006; 441: 523–527.
Buss H, Dorrie A, Schmitz ML, Frank R, Livingstone M, Resch K et al. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem 2004; 279: 49571–49574.
Acknowledgements
This work was supported by grants from Children Leukaemia Research Project, Irish Cancer Society and Health Research Board of Ireland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Rights and permissions
About this article
Cite this article
Naughton, R., Quiney, C., Turner, S. et al. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia 23, 1432–1440 (2009). https://doi.org/10.1038/leu.2009.49
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2009.49
Keywords
This article is cited by
-
Targeting IL-17A enhances imatinib efficacy in Philadelphia chromosome-positive B-cell acute lymphoblastic leukemia
Nature Communications (2024)
-
Targeting PI3K/Akt signaling in prostate cancer therapy
Journal of Cell Communication and Signaling (2023)
-
Context-specific effects of NOX4 inactivation in acute myeloid leukemia (AML)
Journal of Cancer Research and Clinical Oncology (2022)
-
Small-molecule inhibitor targeting the Hsp70-Bim protein–protein interaction in CML cells overcomes BCR-ABL-independent TKI resistance
Leukemia (2021)
-
Combating TKI resistance in CML by inhibiting the PI3K/Akt/mTOR pathway in combination with TKIs: a review
Medical Oncology (2021)